Abstract
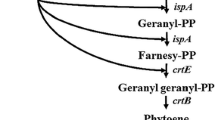
In this study, we used the non-carotenogenic yeast Pichia pastoris X33 as a receptor for β-carotene-encoding genes, in order to obtain new recombinant strains capable of producing different carotenoidic compounds. We designed and constructed two plasmids, pGAPZA-EBI* and pGAPZA-EBI*L*, containing the genes encoding lycopene and β-carotene, respectively. Plasmid pGAPZA-EBI*, expresses three genes, crtE, crtB, and crtI*, that encode three carotenogenic enzymes, geranylgeranyl diphosphate synthase, phytoene synthase, and phytoene desaturase, respectively. The other plasmid, pGAPZA-EBI*L*, carried not only the three genes above mentioned, but also the crtL* gene, that encodes lycopene β-cyclase. The genes crtE, crtB, and crtI were obtained from Erwinia uredovora, whereas crtL* was cloned from Ficus carica (JF279547). The plasmids were integrated into P. pastoris genomic DNA, and the resulting clones Pp-EBI and Pp-EBIL were selected for either lycopene or β-carotene production and purification, respectively. Cells of these strains were investigated for their carotenoid contents in YPD media. These carotenoids produced by the recombinant P. pastoris clones were qualitatively and quantitatively analyzed by high-resolution liquid chromatography, coupled to photodiode array detector. These analyses confirmed that the recombinant P. pastoris clones indeed produced either lycopene or β-carotene, according to the integrated vector, and productions of 1.141 μg of lycopene and 339 μg of β-carotene per gram of cells (dry weight) were achieved. To the best of our knowledge, this is the first time that P. pastoris has been genetically manipulated to produce β-carotene, thus providing an alternative source for large-scale biosynthesis of carotenoids.




Similar content being viewed by others
References
An GH, Shuman DB, Johnson EA (1989) Isolation of Phaffia rhodozyma mutants with increased astaxanthin content. Appl Environ Microbiol 55:116–124
Araya-Garay JM, Feijoo-Siota L, Veiga-Crespo P, Villa TG (2011) cDNA cloning of a novel gene codifying for the enzyme lycopene β-cyclase from Ficus carica and its expression in the Escherichia coli. Appl Microbiol Biotechnol 92:769–777. doi:10.1007/s00253-011-3488-8
Becerra M, Belmonte ER, Cerdán ME, Siso MIG (2001) Extraction of intra cell proteins from Kluyveromyces lactis. Food Technol Biotechnol 39:135–139
Bhataya A, Schmidt-Dannert C, Lee PC (2009) Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem 44:1095–1102
Bhosale P (2004) Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl Microbiol Biotechnol 63:351–361
Buxadó JA, Heynngnezz LE, Juiz AG, Tamayo G, Lima IR, Marshalleck HD, Mola EL (2004) Scale-up of processes to isolate the misstargeted rBm86 protein from Pichia pastoris. Afr J Biotechnol 11:599–605
Buzzini P, Rubinstein L, Martini A (2001) Production of yeast carotenoids by using agro-industrial by-products. Agro Food Ind Hi-Tech 12:7–10
Cereghino GP, Cereghino JL, Ilgen C, Cregg JM (2002) Production of recombinant proteins in fermenter cultures of the yeast Pichia pastoris: heterologous protein expression in the methylotrophic yeast Pichia pastoris. Curr Opin Biotechnol 13(4):329–332
Cheng Q (2006) Structural diversity and functional novelty of new carotenoid biosynthesis genes. J Ind Microbiol Biotechnol 33(7):552–559
Cregg JM, Cereghino JL, Shi J, Higgins DR (2000) Recombinant protein expression in Pichia pastoris. Mol Biotechnol 16:23–52
Cregg JM, Tolstorukov I, Kusari A, Sunga J, Madden K, Chappell T (2009) Expression in the yeast Pichia pastoris. Meth Enzymol 463:169–189. doi:10.1016/S0076-6879(09)63013-5
D’Ambrosio C, Giorio G, Marino I, Merendino A, Petrozza A, Salfi L, Stigliani A, Cellini F (2004) Virtually complete conversion of lycopene into beta-carotene in fruits of tomato plants transformed with the tomato lycopene beta cyclase (tlcy-b) cDNA. Plant Sci 166:207–214. doi:10.1016/j.plantsci.2003.09.015
dos Santos RA, da Silva R, Kalil S, Veiga A, de Fernandes J (2011) Different cell disruption methods for astaxanthin recovery by Phaffia rhodozyma. Afr J Biotechnol 10(7):1165–1171
Fraser PD, Bramley P (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43(3):228–265
Fraser PD, Pinto MES, Holloway DE, Bramley PM (2000) Application of high performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. The Plant J 24:551–558
Frengova GI, Simova D, Beshkova DM (2003) Carotinoid production by lactose-negative yeasts co-cultivated with lactic acid bacteria in whey ultrafiltrate. Zeitschrift für Naturforschung 58:562–567
Jiang Y, Proteaus P, Poulters D, Ferro-Novick S (1995) Bts1 encodes a geranylgeranyl diphosphate synthase in Saccharomyces cerevisiae. J Biol Chem 270(37):21793–21799
Krinsky NI (1994) The biological properties of carotenoids. Pure Appl Chem 66:1003–1010
Kula MR (1990) Trends and future prospects of aqueous two-phase extraction. Bioseparation 1:181–189
Lange N, Steinbüchel A (2011) β-carotene production by Saccharomyces cerevisiae with regard to plasmid stability and culture media. Appl Microbiol Biotechnol 91:1611–1622
Lee PC, Momen AZR, Mijts BN, Schmidt-Dannert C (2003) Biosynthesis of structurally novel carotenoids in Escherichia coli. Chem Biol 10(5):453–462
Lee PC, Salomon C, Mijts B, Schmidt-Dannert C (2008) Biosynthesis of ubiquinone compounds with conjugated prenyl side chains. Appl Environ Microbiol 74(22):6908–6917
Lin Cereghino J, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24:45–66
Liu YS, Wu JY, Ho KP (2006) Characterization of oxygen transfer conditions and their effects on Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. Biochem Eng J 27:331–335
Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22:249–270
Martín J, Gudiña E, Barredo J (2008) Conversion of β-carotene into astaxanthin: two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microbial Cell Factories 7:3. doi:10.1186/1475-2859-7-3
Medeiros FO, Alves FG, Lisboa CR, Martins DS, Burkert CAV, Kalil SJ (2008) Ultrasonic waves and glass pearls: a new method of extraction of beta-galactosidase for use in laboratory. Quim Nova 31:336–339
Meléndez Martínez AJ, Vicario IM, Heredia FJ (2003) A routine high-perfomance liquid chromatography method for carotenoid determination in ultrafrozen orange juices. J Agric Food Chem 51:4219–4224
Misawa N, Shimada H (1998) Metabolic engineering for the production of carotenoids in non-carotenogenic bacteria and yeasts. J Biotechnol 59(3):169–181
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol 177:6575–6584
Miura Y, Kondo K, Saito T, Shimada H, Fraser PD, Misawa N (1998) Production of the carotenoid lycopene, beta-carotene, and astaxanthin in the food yeast Candida utilis. Appl Environ Microbiol 64(4):1226–1229
Papp T, Velayos A, Bartók T, Eslava AP, Vágvölgyi C, Iturriaga E (2006) Heterologous expression of astaxanthin biosynthesis genes in Mucor circinelloides. Appl Microbiol Biotechnol 69:526–531
Rodney AL (1994) Production of carotenoids by recombinant DNA technology. Pure Appl Chem 5(66):1057–1062
Rodrigo MJ, Marcos JF, Zacarías L (2004) Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. J Agric Food Chem 52(22):6724–6731
Sandmann G (1994) Phytoene desaturase—genes, enzymes and phylogenetic aspects. J Plant Physiol 143(4–5):444–447
Sandmann G (2001) Genetic manipulation of carotenoid biosynthesis: strategies, problems and achievements. Trends Plant Sci 6:14–17
Schmidt-Dannert C, Umeno D, Arnold FH (2000) Molecular breeding of carotenoid biosynthetic pathways. Nat Biotechnol 18(7):750–753
Schmidt-Dannert C, Lee PC, Mijts BN (2006) Creating carotenoid diversity in E. coli cells using combinatorial and directed evolution strategies. Phytochem Rev 5(1):67–74
Shimada H, Kondo K, Fraser PD, Miura Y, Saito T, Misawa N (1998) Increased carotenoid production by the food yeast Candida utilis through metabolic engineering of the isoprenoid pathway. Appl Environ Microbiol 64(7):2676–2680
Siefermann-Harms D (1987) The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol Plant 69(3):561–568
Steiger S, Sandmann G (2004) Cloning of two carotenoid ketolase genes from Nostoc punctiforme for the heterologous production of canthaxanthin and astaxanthin. Biotechnol Lett 26:813–817
Ukibe K, Hashida K, Yoshida N, Takagi H (2009) Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl Environ Microbiol. doi:10.1128/AEM.01249-09
Verwaal R, Wang J, Meijnen J-P, Visser H, Sandmann G, van den Berg JA, van Ooyen A (2007) High-level production of beta-carotene in Saccharomyces cereviseae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 73(13):4342–4350
Vik A, Rine J (2001) Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol 21(19):6395–6405
Wang C-W, Oh M-K, Liao JC (1999) Engineered isoprenoid pathway enhances astaxanthin production in Escherichia coli. Biotechnol Bioeng 62:235–241
Yamamo S, Ishii T, Nakagawa M, Ikenaga H, Misawa N (1994) Metabolic engineering for production of beta-carotene and lycopene in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 58(6):1112–1114
Yuan LZ, Rouviere PE, LaRossa RA, Suh W (2006) Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metab Eng 8(1):79–90
Acknowledgments
J. M. A-G. is the recipient of an AECID scholarship from the Spanish Foreign Affairs Ministry. The authors thank Dr. Norihiko Misawa (Research Institute for Bioresources and Biotechnology, Ishikawa Prefectural University) for the gift of plasmid pACCRT-EIB and both the Faculty of Pharmacy and School of Biotechnology for their support throughout this project. The authors also wish to thank Dr. Angeles Sánchez-Pérez, the University of Sydney, for critically reading and editing the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Araya-Garay, J.M., Feijoo-Siota, L., Rosa-dos-Santos, F. et al. Construction of new Pichia pastoris X-33 strains for production of lycopene and β-carotene. Appl Microbiol Biotechnol 93, 2483–2492 (2012). https://doi.org/10.1007/s00253-011-3764-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3764-7