Abstract
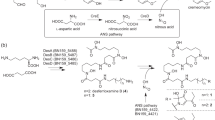
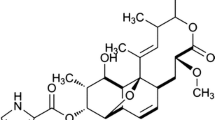
A number of structurally diverse natural products harboring pyrrole moieties possess a wide range of biological activities. Studies on biosynthesis of pyrrole ring have shown that pyrrole moieties are derived from l-proline. Nargenicin A1, a saturated alicyclic polyketide from Nocardia sp. CS682, is a pyrrole-2-carboxylate ester of nodusmicin. We cloned and identified a set of four genes from Nocardia sp. CS682 that show sequence similarity to the respective genes involved in the biosynthesis of the pyrrole moieties of pyoluteorin in Pseudomonas fluorescens, clorobiocin in Streptomyces roseochromogenes subsp. Oscitans, coumermycin A1 in Streptomyces rishiriensis, one of the pyrrole rings of undecylprodigiosin in Streptomyces coelicolor, and leupyrrins in Sorangium cellulosum. These genes were designated as ngnN4, ngnN5, ngnN3, and ngnN2. In this study, we presented the evidences that the pyrrole moiety of nargenicin A1 was also derived from l-proline by the coordinated action of three proteins, NgnN4 (proline adenyltransferase), NgnN5 (proline carrier protein), and NgnN3 (flavine-dependent acyl-coenzyme A dehydrogenases). Biosynthesis of pyrrole moiety in nargenicin A1 is initiated by NgnN4 that catalyzes ATP-dependent activation of l-proline into l-prolyl-AMP, and the latter is transferred to NgnN5 to create prolyl-S-peptidyl carrier protein (PCP). Later, NgnN3 catalyzes the two-step oxidation of prolyl-S-PCP into pyrrole-2-carboxylate. Thus, this study presents another example of a pyrrole moiety biosynthetic pathway that uses a set of three genes to convert l-proline into pyrrole-2-carboxylic acid moiety.







Similar content being viewed by others
References
Celmer WD, Chmurny GN, Moppett CE, Ware RS, Watts PC, Whipple EB (1980) Structure of natural antibiotic CP-47,444. J Am Chem Soc 102:4203–4209
Challis GL, Ravel J, Townsend CA (2000) Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem Biol 7:211–244
Ehmann DE, Shaw-Reid CA, Losey HC, Walsh CT (2000) The EntF and EntE adenylation domains of Escherichia coli enterobactin synthetase: sequestration and selectivity in acyl-AMP transfers to thiolation domain cosubstrates. Proc Natl Acad Sci 97:2509–2514
Feitelson JS, Malpartida F, Hopwood DA (1985) Genetic and biochemical characterization of the red gene cluster of Streptomyces coelicolor A3(2). J Gen Microbiol 131:2431–2441
Garneau S, Dorrestein PC, Kelleher NL, Walsh CT (2005) Characterization of the formation of the pyrrole moiety during clorobiocin and coumermycin A1 biosynthesis. Biochemistry 44:2770–2780
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical streptomyces genetics. John Innes Foundation, Norwich
Kopp M, Irschik H, Gemperlein K, Buntin K, Meiser P, Weissman KJ, Bode HB, Muller R (2011) Insights into the complex biosynthesis of the leupyrrins in Sorangium cellulosum So ce690. Mol Biosyst 7:1549–1563
Mootz HD, Marahiel MA (1997) The tyrocidine biosynthesis operon of Bacillus brevis: complete nucleotide sequence and biochemical characterization of functional internal adenylation domains. J Bacteriol 179:6843–6850
Nowak-Thompson B, Chane N, Wing JS, Gould SJ, Loper JE (1999) Characterization of the pyoluteorin biosynthetic gene cluster of Pseudomonas fluorescens Pf-5. J Bacteriol 181:2166–2174
Pojer F, Li SM, Heide L (2002) Molecular cloning and sequence analysis of the clorobiocin biosynthetic gene cluster: new insights into the biosynthesis of aminocoumarin antibiotics. Microbiology 148:3901–3911
Quadri LE, Weinreb PH, Lei M, Nakano MM, Zuber P, Walsh CT (1998) Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 37:1585–1595
Rosa DC, Oletta CA, Zlotnik H (1996) A rapid and gentle method for isolation of genomic DNA from pathogenic Nocardia sp. Clin Diagn Lab Immunol 601–604
Sali A, Blundell TL (1993) Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Seung SC, Sohng JK, Lee HJ, Park SJ, Simkhada JR, Yoo JC (2009a) Quantitative analysis of nargenicin in Nocardia sp. CS682 culture by high performance liquid chromatography. Arch Pharm Res 32:335–340
Seung HK, Yoo JC, Kim TS (2009b) Nargenicin enhances 1,25-dihydroxyvitamin D3- and all-trans retinoic acid-induced leukemia cell differentiation via PKCbI/MAPK pathways. Biochem Pharmacol 77:1694–1701
Sherman DH, Li S, Yermalitskaya LV, Kim Y, Smith JA, Waterman MR (2006) The structural basis for substrate anchoring, active site selectivity, and product formation by P450 PikC from Streptomyces venezuelae. J Biol Chem 281:26289–26297
Sippl MJ (1993) Recognition of errors in three-dimensional structure of protein. Protein 17:355–362
Sohng JK, Yamaguchi T, Seong CN, Baik KS, Park SC, Lee HJ, Jang SY, Simkhada JR, Yoo JC (2008) Production, isolation and biological activity of nargenicin from Nocardia sp. CS682. Arch Pharm Res 31:1339–1345
Stachelhaus T, Mootz HD, Marahiel MA (1999) The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem Biol 6:493–505
Thomas MG, Burkart MD, Walsh CT (2002) Conversion of l-proline to pyrrolyl-2-S-PCP during undecylprodigiosin and pyoluteorin biosynthesis. Chem Biol 9:171–184
Venkatachalam CM, Jiang X, Oldfield T, Waldman M (2003) LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J Mol Graph Model 21:289–307
Walsh CT, Garneau-Tsodikova S, Howard-Jones AR (2006) Biological formation of pyrroles: nature’s logic and enzymatic machinery. Nat Prod Rep 23:517–531
Wang ZX, Li SM, Heide L (2000) Identification of the coumermycin A1 biosynthetic gene cluster of Streptomyces rishiriensis DSM 40489. Antimicrob Agents Chemother 44:3040–3048
Weinreb PH, Quadri LEN, Walsh CT, Zuber P (1998) Stoichiometry and specificity of in vitro phosphopantetheinylation and aminoacylation of the valine-activating module of surfactin synthetase. Biochemistry 37:1575–1584
Xu H, Wang ZX, Schmidt J, Heide L, Li SM (2002) Genetic analysis of the biosynthesis of the pyrrole and carbamoyl moieties of coumermycin A1 and novobiocin. Mol Genet Genomics 268:387–396
Acknowledgments
This study was supported by Technology Development Program for Agriculture and Forestry, Ministry of Agriculture and Forestry (20100368), Republic of Korea, 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maharjan, S., Aryal, N., Bhattarai, S. et al. Biosynthesis of the nargenicin A1 pyrrole moiety from Nocardia sp. CS682. Appl Microbiol Biotechnol 93, 687–696 (2012). https://doi.org/10.1007/s00253-011-3567-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3567-x