Abstract
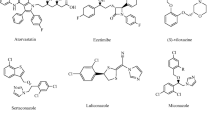
The enzymatic preparation of optically pure tertiary alcohols under sustainable conditions has received much attention. The conventional chemical synthesis of these valuable building blocks is still hampered by the use of harmful reagents such as heavy metal catalysts. Successful examples in biocatalysis used esterases, lipases, epoxide hydrolases, halohydrin dehalogenases, thiamine diphosphate-dependent enzymes, terpene cyclases, -acetylases, and -dehydratases. This mini-review provides an overview on recent developments in the discovery of new enzymes, their functional improvement by protein engineering, the design of chemoenzymatic routes leading to tertiary alcohols, and the discovery of entirely new biotransformations.












Similar content being viewed by others
References
Agger SA, Lopez-Gallego F, Hoye TR, Schmidt-Dannert C (2008) Identification of sesquiterpene synthases from Nostoc punctiforme PCC 73102 and Nostoc sp. strain PCC 7120. J Bacteriol 190:6084–6096
Ajikumar PK, Xiao WH, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science 330:70–74
Andexer JN, Langermann JV, Kragl U, Pohl M (2009) How to overcome limitations in biotechnological processes—examples from hydroxynitrile lyase applications. Trends Biotechnol 27:599–607
Avi M, Fechter MH, Gruber K, Bela F, Pochlauer P, Griengl H (2004) Hydroxynitrile lyase catalysed synthesis of heterocyclic (R)- and (S)-cyanohydrins. Tetrahedron 60:10411–10418
Bartsch S, Kourist R, Bornscheuer UT (2008) Complete inversion of enantioselectivity towards acetylated tertiary alcohols by a double mutant of a Bacillus subtilis esterase. Angew Chem Int Ed 47:1508–1511
Bassegoda A, Nguyen GS, Schmidt M, Kourist R, Diaz P, Bornscheuer UT (2010) Rational protein design of Paenibacillus barcinonensis esterase EstA for kinetic resolution of tertiary alcohols. ChemCatChem 2:962–967
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci U S A 95:4126–4133
Bosley JA, Casey J, Macrae AR, MyCock G (1994) Process for the esterification of carboxylic acids with tertiary alcohols. WO 95/01450
Brodkorb D, Harder J (2009) First enzymatic insight in anaerobic monoterpenes degradation. Characterization of the first initial steps. Biotrans 2009. Conference proceedings
Brodkorb D, Gottschall M, Marmulla R, Luddeke F, Harder J (2010) Linalool dehydratase-isomerase, a bifunctional enzyme in the anaerobic degradation of monoterpenes. J Biol Chem 285:30436–30442
Cane DE, Watt RM (2003) Expression and mechanistic analysis of a germacradienol synthase from Streptomyces coelicolor implicated in geosmin biosynthesis. Proc Natl Acad Sci U S A 100:1547–1551
Choi WJ (2009) Biotechnological production of enantiopure epoxides by enzymatic kinetic resolution. Appl Microbiol Biotechnol 84:239–247
Christoffers J, Baro A (2005) Quaternary stereocenters, challenges and solutions for organic synthesis. Wiley-VCH, Weinheim
Chipman D, Barak Z, Schloss JV (1998) Biosynthesis of 2-aceto-2-hydroxy acids: acetolactate synthases and acetohydroxyacid synthases. Biochim Biophys Act 1385:401–419
Cozzi PG, Hilgraf R, Zimmermann N (2007) Enantioselective catalytic formation of quaternary stereogenic centers. Eur J Org Chem 2007(36):5969–5994
Crowell AL, Williams DC, Davis EM, Wildung MR, Croteau R (2002) Molecular cloning and characterization of a new linalool synthase. Arch Biochem Biophys 405:112–121
Degenhardt J, Kollner TG, Gershenzon J (2009) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70:1621–1637
Dominguez de Maria P, Carboni-Oerlemans C, Tuin B, Bargeman G, van der Meer A, van Gemert R (2005) Biotechnological applications of Candida antarctica lipase A: state-of-the-art. J Mol Catal B Enzym 37:36–46
Enders D, Balensiefer T, Niemeier O, Christmann M (2007) Enantioselective organocatalysis. Wiley-VCH, Weinheim
Ericsson DJ, Kasrayan A, Johanssonl P, Bergfors T, Sandström AG, Bäckvall JE, Mowbray SL (2008) X-ray structure of Candida antarctica lipase A shows a novel lid structure and a likely mode of interfacial activation. J Mol Biol 376:109–119
Fechter MH, Gruber K, Avi M, Skranc W, Schuster C, Pochlauer P, Klepp KO, Griengl H (2007) Stereoselective biocatalytic synthesis of (S)-2-hydroxy-2-methylbutyric acid via substrate engineering by using “thio-disguised” precursors and oxynitrilase catalysis. Chem Eur J 13:3369–3376
Gall M, Kourist R, Schmidt M, Bornscheuer UT (2010) The role of the GGGX motif in determining the activity and enantioselectivity of pig liver esterase towards tertiary alcohols. Biocatal Biotransform 28:201–208
Hasegawa Y, Gridnev ID, Ikariya T (2010) Enantioselective and Z/E-selective conjugate addition of alpha-substituted cyanoacetates to acetylenic esters catalyzed by bifunctional ruthenium and iridium complexes. Angew Chem Int Ed 49:8157–8160
Heinze B, Kourist R, Fransson L, Hult K, Bornscheuer UT (2007) Highly enantioselective kinetic resolution of a tertiary alcohol using mutants of an esterase from Bacillus subtilis. Prot Eng Des Sel 20:125–131
Hellström H, Steinreiber A, Mayer SF, Faber K (2001) Bacterial epoxide hydrolase-catalyzed resolution of a 2,2-disubstituted oxirane: optimization and upscaling. Biotechnol Lett 23:169–173
Henke E, Pleiss J, Bornscheuer UT (2002) Activity of lipases and esterases towards tertiary alcohols: insights into structure-function relationships. Angew Chem Int Ed 41:3211–3213
Henke E, Bornscheuer UT, Schmid RD, Pleiss J (2003) A molecular mechanism of enantiorecognition of tertiary alcohols by carboxylesterases. Chembiochem 4:485–493
Herter S, Nguyen GS, Thompson ML, Steffen-Munsberg F, Schauer F, Bornscheuer UT, Kourist R (2011) Comparative analysis of tertiary alcohol esterase activity in bacterial strains isolated from enrichment cultures and from screening strain libraries. Appl Microbiol Biotechnol 90:929–939
Hiseni A, Arends IW, Otten LG (2011) Biochemical characterization of the carotenoid 1,2-hydratases (CrtC) from Rubrivivax gelatinosus and Thiocapsa roseopersicina. Appl Microbiol Biotechnol. doi:10.1007/s00253-011-3324-1
Holt J, Hanefeld U (2009) Enantioselective enzyme-catalysed synthesis of cynohydrins. Curr Org Synth 6:15–37
Holt J, Arends IWCE, Minnaard A, Hanefeld U (2007) Hydrolase-catalysed preparation of chiral alpha, alpha-disubstituted cyanohydrin acetates. Adv Synth Catal 349:1341–1344
Hotta Y, Ezaki S, Atomi H, Imanaka T (2002) Extremely stable and versatile carboxylesterase from a hyperthermophilic archaeon. Appl Environ Microbiol 68:3925–3931
Hou CT, Adachi S (2005) Handbook of industrial biocatalysis. CRC Taylor & Francis, Boca Raton
Komatsu M, Tsuda M, Omura S, Oikawa H, Ikeda H (2008) Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc Natl Acad Sci U S A 105:7422–7427
Kourist R, Bartsch S, Bornscheuer UT (2007a) Highly enantioselective synthesis of arylaliphatic tertiary alcohols using mutants of an esterase from Bacillus subtilis. Adv Synth Catal 349:1393–1398
Kourist R, Krishna SH, Patel JS, Bartnek F, Hitchman TS, Weiner DP, Bornscheuer UT (2007b) Identification of a metagenome-derived esterase with high enantioselectivity in the kinetic resolution of arylaliphatic tertiary alcohols. Org Biomol Chem 5:3310–3313
Kourist R, Dominguez de Maria P, Bornscheuer UT (2008a) Enzymatic synthesis of optically active tertiary alcohols: expanding the biocatalysis toolbox. Chembiochem 9:491–498
Kourist R, Nguyen GS, Strübing D, Böttcher D, Liebeton K, Naumer C, Eck J, Bornscheuer UT (2008b) Hydrolase-catalyzed stereoselective preparation of protected alpha, alpha-dialkyl-alpha-hydroxycarboxylic acids. Tetrahedron Asymmetry 19:1839–1843
Kourist R, Jochens H, Bartsch S, Kuipers R, Kumar Padhi S, Gall M, Böttcher D, Joosten H-J, Bornscheuer UT (2010a) The alpha/beta-hydrolase fold 3DM database (ABHDB) as a tool for protein engineering. Chembiochem 11:1635–1643
Kourist R, Brundiek H, Bornscheuer UT (2010b) Protein engineering and discovery of lipases. Eur J Lipid Sci Technol 112:64–74
Krishna SH, Persson M, Bornscheuer UT (2002) Enantioselective transesterification of a tertiary alcohol by lipase A from Candida antarctica. Tetrahedron Asymmetry 13:2693–2696
Landmann C, Fink B, Festner M, Dregus M, Engel KH, Schwab W (2007) Cloning and functional characterization of three terpene synthases from lavender (Lavandula angustifolia). Arch Biochem Biophys 465:417–429
Larkov O, Zaks A, Bar E, Lewinsohn E, Dudai N, Mayer AM, Ravid U (2008) Enantioselective monoterpene alcohol acetylation in Origanum, Mentha and Salvia species. Phytochemistry 69:2565–2571
Lehwald P, Richter M, Rohr C, Liu HW, Müller M (2010) Enantioselective intermolecular aldehyde-ketone cross-coupling through an enzymatic carboligation reaction. Ang Chem Int Ed 49:2389–2392
Lutz S, Bornscheuer UT (eds) (2008) Protein engineering handbook. Wiley-VCH, Weinheim
Majeric-Elenkov M, Hoeffken HW, Tang L, Hauer B, Janssen DB (2007) Enzyme-catalyzed nucleophilic ring opening of epoxides for the preparation of enantiopure tertiary alcohols. Adv Synth Catal 349:2279–2285
Majeric-Elenkov M, Tang LX, Meetsma A, Hauer B, Janssen DB (2008) Formation of enantiopure 5-substituted oxazolidinones through enzyme-catalysed kinetic resolution of epoxides. Org Lett 10:2417–2420
Mihovilovic MD, Leisch HG, Mereiter K (2004) Microwave-mediated intramolecular Diels–Alder cyclization of biodihydroxylated benzoic acid derivatives. Tetrahedron Lett 45:7087–7090
Molinaro C, Guilbault AA, Kosjek B (2010) Resolution of 2,2-disubstituted epoxides via biocatalytic azidolysis. Org Lett 12:3772–3775
Nguyen GS, Kourist R, Paravidino M, Hummel A, Rehdorf J, Orru RVA, Hanefeld U, Bornscheuer UT (2010) An enzymatic toolbox for the kinetic resolution of 2-(pyridinyl)but-3-yn-2-ols and tertiary cyanohydrins. Eur J Org Chem 14:2753–2758
O'Hagan D, Zaidi NA (1992) Hydrolytic resolution of tertiary acetylenic acetate esters with the lipase from Candida cylindracea. J Chem Soc Perkin Trans 1:947–949
Özdemirhan D, Sezer S, Sonmez Y (2008) Enzyme-catalyzed resolution of aromatic ring fused cyclic tertiary alcohols. Tetrahedron Asymmetry 19:2717–2720
Paravidino M, Holt J, Romano D, Singh N, Arends I, Minnaard AJ, Orru RVA, Molinari F, Hanefeld U (2010) Microbial-catalysed resolution of sterically demanding cyanohydrins. J Mol Catal B Enzym 63:87–92
Pleiss J, Fischer M, Peiker M, Thiele C, Schmid RD (2000) Lipase engineering database—understanding and exploiting sequence-structure-function relationships. J Mol Catal B Enzym 10:491–508
Pogorevc M, Faber K (2000) Biocatalytic resolution of sterically hindered alcohols, carboxylic acids and esters containing fully substituted chiral centers by hydrolytic enzymes. J Mol Catal B Enzym 10:357–376
Purkarthofer T, Skranc W, Schuster C, Griengl H (2007) Potential and capabilities of hydroxynitrile lyases as biocatalysts in the chemical industry. Appl Microbiol Biotechnol 76:309–320
Rashamuse K, Magomani V, Ronneburg T, Brady D (2009) A novel family VIII carboxylesterase derived from a leachate metagenome library exhibits promiscuous beta-lactamase activity on nitrocefin. Appl Microbiol Biotechnol 83:491–500
Seebach D (2011) Generation of secondary, tertiary, and quaternary centers by geminal disubstitution of carbonyl oxygens. Angew Chem Int Ed 50:96–101
Sonawane RP, Jheengut V, Rabalakos C, Larouche-Gauthier R, Scott HK, Aggarwal VK (2011) Enantioselective construction of quaternary stereogenic centers from tertiary boronic esters: methodology and applications. Angew Chem Int Ed 50:3760–3763
Steinreiber A, Faber K (2001) Microbial epoxide hydrolases for preparative biotransformations. Curr Opin Biotechnol 12:552–558
Stymiest JL, Bagutski V, French RM, Aggarwal VK (2008) Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 456:778–783
Wiggers M, Holt J, Kourist R, Bartsch S, Arends I, Minnaard AJ, Bornscheuer UT, Hanefeld U (2009) Probing the enantioselectivity of Bacillus subtilis esterase BS2 for tert. alcohols. J Mol Catal B Enzym 60:82–86
Yoshikuni Y, Martin VJ, Ferrin TE, Keasling JD (2006) Engineering cotton (+)-delta-cadinene synthase to an altered function: germacrene D-4-ol synthase. Chem Biol 13:91–98
Acknowledgments
RK is grateful to the “Fonds der Chemischen Industrie” (Frankfurt, Germany) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kourist, R., Bornscheuer, U.T. Biocatalytic synthesis of optically active tertiary alcohols. Appl Microbiol Biotechnol 91, 505–517 (2011). https://doi.org/10.1007/s00253-011-3418-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3418-9