Abstract
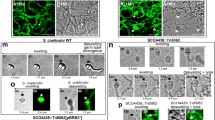
In Streptomyces coelicolor, the sco2127 gene is located upstream of the gene encoding for glucose kinase. This region restores sensitivity to carbon catabolite repression (CCR) of Streptomyces peucetius var. caesius mutants, resistant to 2-deoxyglucose (DogR). In order to search for the possible mechanisms behind this effect, sco2127 was overexpressed and purified for protein–protein interaction studies. SCO2127 was detected during the late growth phase of S. coelicolor grown in a complex media supplemented with 100 mM glucose. Pull-down assays using crude extracts from S. coelicolor grown in the same media, followed by far-western blotting, allowed detection of two proteins bound to SCO2127. The proteins were identified by MALDI-TOF mass spectrometry as SCO5113 and SCO2582. SCO5113 (BldKB) is a lipoprotein ABC-type permease (∼66 kDa) involved in mycelium differentiation by allowing the transport of the morphogenic oligopeptide Bld261. SCO2582, is a putative membrane metalloendopeptidase (∼44 kDa) of unknown function. In agreement with the possible role of SCO2127 in mycelium differentiation, delayed aerial mycelium septation and sporulation was observed when S. coelicolor A3(2) was grown in the presence of elevated glucose concentrations (100 mM), an effect not seen in a Δ-sco2127 mutant derived from it. We speculate that SCO2127 might represent a key factor in CCR of mycelium differentiation by interacting with BldKB.



Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Angell S, Schwartz E, Bibb JM (1992) The glucose kinase gene of Streptomyces coelicolor A3(2): its nucleotide sequence, transcriptional analysis and role in glucose repression. Mol Microbiol 6:2833–2844
Angell S, Lewis CG, Buttner MJ, Bibb JM (1994) Glucose repression in Streptomyces coelicolor A3(2): a likely regulatory role for glucose kinase. Mol Gen Genet 244:135–143
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Bertram R, Schlicht M, Mahr K, Nothaft H, Saier MH Jr, Titgemeyer F (2004) In silico and transcriptional analysis of carbohydrate uptake systems of Streptomyces coelicolor A3(2). J Bacteriol 186:1362–1373
Bierman M, Logan R, O’Brien K, Seno ET, Nagaraja Rao R, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49
Chávez A, García-Huante Y, Ruiz B, Langley E, Rodríguez-Sanoja R, Sanchez S (2009) Cloning and expression of the sco2127 gene from Streptomyces coelicolor M145. J Ind Microbiol Biotechnol 36:649–654
Claessen D, de Jong W, Dijkhuizen L, Wösten HA (2006) Regulation of Streptomyces development: reach for the sky! Trends Microbiol 14:313–319
Edmonson DG, Dent SYR (2001) Identification of protein interactions by far western analysis. In: Coligan JE, Dunn BM, Ploegh HL, Speicher DW, Wingfield PT (eds) Current protocols in protein science. Wiley, New York, pp 19.7.1–19.7.10
Escalante L, Ramos I, Imriskova I, Langley E, Sanchez S (1999) Glucose repression of anthracycline formation in Streptomyces peucetius var. caesius. Appl Microbiol Biotechnol 52:572–578
Gottesman S (1999) Regulation by proteolysis: developmental switches. Curr Opin Microbiol 2:142–147
Gust B, Kieser T, Chater KF (2002) REDIRECT© technology: PCR-targeting system in Streptomyces coelicolor. John Innes Centre, Norwich
Guzmán S, Carmona A, López R, Escalante L, Ruiz B, Rodríguez-Sanoja R, Sanchez S, Langley E (2005a) Pleiotropic effect of the SCO2127 gene on the glucose uptake, glucose kinase activity and carbon catabolite repression in Streptomyces peucetius var. caesius. Microbiology-SGM 151:1717–1723
Guzmán S, Ramos I, Moreno E, Ruiz B, Rodríguez-Sanoja R, Escalante L, Langley E, Sanchez S (2005b) Sugar uptake and sensitivity to carbon catabolite repression in Streptomyces peucetius var. caesius. Appl Microbiol Biotechnol 69:200–206
Henzel WJ, Stults JT (1996) Unit 16.2. Matrix assisted laser desorption/ionization time-of-flight mass analysis of peptides. In: Coligan JE, Dunn BM, Ploegh HL, Speicher DW, Wingfield PT (eds) Current protocols in protein science. Wiley, New York, pp 16.1.1–16.2.11
Hillerich B, Westpheling J (2006) A new GntR family transcriptional regulator in Streptomyces coelicolor is required for morphogenesis and antibiotic production and controls transcription of an ABC transporter in response to carbon source. J Bacteriol 188:7477–7487
Hodgson D (1982) Glucose repression of carbon source uptake and metabolism in Streptomyces coelicolor and its perturbation in mutants resistant to 2-deoxyglucose. J Gen Microbiol 128:2417–2430
Holland IB, Blight MA (1999) ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J Mol Biol 293:381–399
Hu YX, Guo JY, Shen L, Chen Y, Zhang ZC, Zhang YL (2002) Get effective polyclonal antisera in one month. Cell Res 12:157–160
Ikeda H, Seno ET, Bruton CJ, Chater KF (1984) Genetic mapping, cloning and physiological aspects of the glucose kinase gene of Streptomyces coelicolor. Mol Gen Genet 196:501–507
Jensen ON, Larsen MR, Roepstorff P (1998) Mass spectrometric identification and microcharacterization of proteins from electrophoretic gels: strategies and applications. Proteins 2:74–89
Kurisu G, Kinoshita T, Sugimoto A, Nagara A, Kai Y, Kasai N, Harada S (1997) Structure of the zinc endoprotease from Streptomyces caespitosus. J Biochem (Tokyo) 121:304–308
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:6801–6805
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Nodwell J, Losick R (1998) Purification of an extracellular signaling molecule involved in production of aerial mycelium by Streptomyces coelicolor. J Bacteriol 180:1334–1337
Okamoto S, Lezhava A, Hosaka T, Okamoto-Hosoya Y, Ochi K (2003) Enhanced expression of S-adenosylmethionine synthetase causes overproduction of actinorhodin in Streptomyces coelicolor A3(2). J Bacteriol 185:601–609
Ramos I, Guzmán S, Escalante L, Imriskova I, Rodríguez-Sanoja R, Sanchez S, Langley E (2004) The glucose kinase alone cannot be responsible for carbon source regulation in Streptomyces peucetius var. caesius. Res Microbiol 155:267–274
Segura D, Gonzalez R, Rodriguez-Sanoja R, Sandoval T, Escalante L, Sanchez S (1996) Streptomyces mutants insensitive to glucose repression showed deregulation of primary and secondary metabolism. Asia Pac J Mol Biol Biotechnol 4:30–36
Shin S-K, Park H-S, Kwon H-J, Yoon H-J, Suh J-W (2007) Genetic characterization of two S-adenosylmethionine-induced ABC transporter reveals their roles in modulations of secondary metabolism and sporulation in Streptomyces coelicolor M145. J Microbiol Biotechnol 17:1818–1825
Ueda K, Endo K, Takano H, Nishimoto M, Kido Y, Tomaru Y, Matsuda K, Beppu T (2000) Carbon-source-dependent transcriptional control involved in the initiation of cellular differentiation in Streptomyces griseus. Anton Leeuw Int J G 78:263–268
Acknowledgements
We are indebted to M. A. Ortíz and L. Escalante for their assistance during the elaboration of this work. This work was supported in part by the grants IN 209210 from PAPIIT, Dirección General de Asuntos del Personal Académico, UNAM and CB2008-100564-IIBO from Consejo Nacional de Ciencia y Tecnología, Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chávez, A., Forero, A., Sánchez, M. et al. Interaction of SCO2127 with BldKB and its possible connection to carbon catabolite regulation of morphological differentiation in Streptomyces coelicolor . Appl Microbiol Biotechnol 89, 799–806 (2011). https://doi.org/10.1007/s00253-010-2905-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2905-8