Abstract
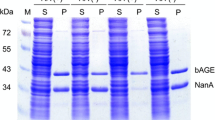
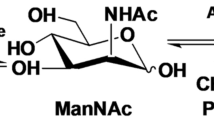
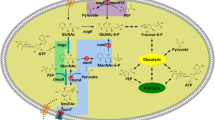
N-Acetyl-d-neuraminic acid (Neu5Ac) is a precursor for producing many pharmaceutical drugs such as zanamivir which have been used in clinical trials to treat and prevent the infection with influenza virus, such as the avian influenza virus H5N1 and the current 2009 H1N1. Two recombinant Escherichia coli strains capable of expressing N-acetyl-d-glucosamine 2-epimerase and N-acetyl-d-neuraminic acid aldolase were constructed based on a highly efficient temperature-responsive expression system which is safe compared to chemical-induced systems and coupled in Neu5Ac production. Carbon sources were optimized for Neu5Ac production, and the concentration effects of carbon sources on the production were investigated. With 2,200 mM pyruvate as carbon source and substrate, 61.9 mM (19.1 g l−1) Neu5Ac was produced from 200 mM N-acetyl-d-glucosamine (GlcNAc) in 36 h by the coupled cells. Our Neu5Ac biosynthetic process is favorable compared with natural product extraction, chemical synthesis, or even many other biocatalysis processes.







Similar content being viewed by others
References
Aisaka K, Uwajima T (1986) Cloning and constitutive expression of the N-acetylneuraminate lyase gene of Escherichia coli. Appl Environ Microbiol 51:562–565
Blattner FR, Plunkett G 3rd, Bloch CA et al (1997) The complete genome sequence of Escherichia coli K-12. Science 277:1453–1474
Booth IR (1985) Regulation of cytoplasmic pH in bacteria. Microbiol Rev 49:359–378
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chambers SP, Prior SE, Evans RA et al (1988) Plasmid pMTL153: a high copy number version of pAT153 and its use to obtain high expression of the Pseudomonas carboxypeptidase G2 gene. Appl Microbiol Biotechnol 29:572–578
Chen Y, Xing D, Wang W et al (2007) Development of an ion-pair HPLC method for investigation of energy charge changes in cerebral ischemia of mice and hypoxia of Neuro-2a cell line. Biomed Chromatogr 21:628–634
Dreyfuss G, Adam SA, Choi YD (1984) Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol 4:415–423
Duetz WA, van Beilen JB, Witholt B (2001) Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr Opin Biotechnol 12:419–425
el-Mansi EM, Holms WH (1989) Control of carbon flux to acetate excretion during growth of Escherichia coli in batch and continuous cultures. J Gen Microbiol 135:2875–2883
el-Mansi EM, Nimmo HG, Holms WH (1986) Pyruvate metabolism and the phosphorylation state of isocitrate dehydrogenase in Escherichia coli. J Gen Microbiol 132:797–806
Furch T, Wittmann C, Wang W et al (2007) Effect of different carbon sources on central metabolic fluxes and the recombinant production of a hydrolase from Thermobifida fusca in Bacillus megaterium. J Biotechnol 132:385–394
Hannig G, Makrides SC (1998) Strategies for optimizing heterologous protein expression in Escherichia coli. Trends Biotechnol 16:54–60
Kawai N, Ikematsu H, Iwaki N et al (2009) Comparison of the effectiveness of zanamivir and oseltamivir against influenza A/H1N1, A/H3N2, and B. Clin Infect Dis 48:996–997
Kilbourne ED (2006) Influenza pandemics of the 20th century. Emerg Infect Dis 12:9–14
Knodler LA, Sekyere EO, Stewart TS et al (1998) Cloning and expression of a prokaryotic enzyme, arginine deiminase, from a primitive eukaryote Giardia intestinalis. J Biol Chem 273:4470–4477
Lee JO, Yi JK, Lee SG et al (2004) Production of N-acetylneuraminic acid from N-acetylglucosamine and pyruvate using recombinant human renin binding protein and sialic acid aldolase in one pot. Enzyme Microb Technol 35:121–125
Lee YC, Chien HC, Hsu WH (2007) Production of N-acetyl-D-neuraminic acid by recombinant whole cells expressing Anabaena sp. CH1 N-acetyl-D-glucosamine 2-epimerase and Escherichia coli N-acetyl-D-neuraminic acid lyase. J Biotechnol 129:453–460
Mahmoudian M, Noble D, Drake CS et al (1997) An efficient process for production of N-acetylneuraminic acid using N-acetylneuraminic acid aldolase. Enzyme Microb Technol 20:393–400
Maru I, Ohnishi J, Ohta Y et al (1998) Simple and large-scale production of N-acetylneuraminic acid from N-acetyl-D-glucosamine and pyruvate using N-acyl-D-glucosamine 2-epimerase and N-acetylneuraminate lyase. Carbohydr Res 306:575–578
Maru I, Ohnishi J, Ohta Y et al (2002) Why is sialic acid attracting interest now? Complete enzymatic synthesis of sialic acid with N-acylglucosamine 2-epimerase. J Biosci Bioeng 93:258–265
Miller LH (1992) A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor
Oxford JS (2000) Influenza A pandemics of the 20th century with special reference to 1918: virology, pathology and epidemiology. Rev Med Virol 10:119–133
Purintrapiban J, Wang MC, Forsberg NE (2003) Degradation of sarcomeric and cytoskeletal proteins in cultured skeletal muscle cells. Comp Biochem Physiol B Biochem Mol Biol 136:393–401
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Schmid A, Dordick JS, Hauer B et al (2001) Industrial biocatalysis today and tomorrow. Nature 409:258–268
Schoemaker HE, Mink D, Wubbolts MG (2003) Dispelling the myths—biocatalysis in industrial synthesis. Science 299:1694–1697
Soundararajan V, Tharakaraman K, Raman R et al (2009) Extrapolating from sequence—the 2009 H1N1 ‘swine’ influenza virus. Nat Biotechnol 27:510–513
Tabata K, Koizumi S, Endo T et al (2002) Production of N-acetyl-D-neuraminic acid by coupling bacteria expressing N-acetyl-D-glucosamine 2-epimerase and N-acetyl-D-neuraminic acid synthetase. Enzyme Microb Technol 30:327–333
von Itzstein M (2007) The war against influenza: discovery and development of sialidase inhibitors. Nat Rev Drug Discov 6:967–974
von Itzstein M, Wu WY, Kok GB et al (1993) Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–423
Wan Y, Xue X, Li M et al (2007) Prepared and screened a modified TNF-alpha molecule as TNF-alpha autovaccine to treat LPS induced endotoxic shock and TNF-alpha induced cachexia in mouse. Cell Immunol 246:55–64
Xu P, Qiu JH, Zhang YN et al (2007) Efficient whole-cell biocatalytic synthesis of N-acetyl-D-neuraminic acid. Adv Synth Catal 349:1614–1618
Zhang Z, Yao L, Hou Y (1990) Construction and application of high level expression vector containing PR PL promoter. Chin J Virol 6:111–116
Zhang JF, Shi ZK, Kong FK et al (2006) Topical application of Escherichia coli-vectored vaccine as a simple method for eliciting protective immunity. Infect Immun 74:3607–3617
Acknowledgments
The work was supported in part by the grants from the State Major Basic Research Development Program (China; numbers 2007CB714303 and 2007CB707803). The authors would also like to acknowledge the partial financial supports from National Natural Science Foundation of China. Vector pBV220 was a kind gift from Prof. G. Wang.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Y., Tao, F., Du, M. et al. An efficient method for N-acetyl-d-neuraminic acid production using coupled bacterial cells with a safe temperature-induced system. Appl Microbiol Biotechnol 86, 481–489 (2010). https://doi.org/10.1007/s00253-009-2302-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2302-3