Abstract
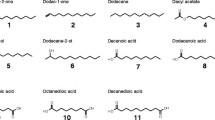
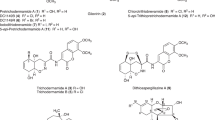
In this study, we investigated the ability of the yeast Trichosporon asahii SBUG-Y 833 to assimilate phenylalkanes with alkyl chain lengths from 7 to 12 carbon atoms, and we describe for the first time the formation of coumarines via a novel degradation pathway other than the normal terminal and ß-oxidation pathway of the alkyl residues. Besides benzoic acid and its further oxidation products, six new metabolites were identified. These were the three coumarines—4-hydroxycoumarin, 4,6-dihydroxycoumarin, 4,8-dihydroxycoumarin—and the three alkyl substituted aromatic acids—7-phenylheptanoic acid, 2-hydroxyphenylheptanoic acid, and 2-hydroxyphenylpropanoic acid. 4-Hydroxycoumarin was the main extracellular metabolite during the degradation of both odd- and even-chain phenylalkanes and was also produced during further biotransformation of 2-hydroxyphenylpropanoic acid and trans-2-hydroxycinnamic acid. Due to the ability of T. asahii to form hydroxylated coumarines, the transformation of 7-hydroxycoumarin and 2,4-dihydroxyphenylpropanoic acid was investigated. Yeast cells supplemented with 7-hydroxycoumarin formed 6,7-dihydroxycoumarin and 4,7-dihydroxycoumarin. The transformation of 2,4-dihydroxyphenylpropanoic acid yielded to 4,7-dihydroxycoumarin as the main product. All hydroxylated coumarines were continuously accumulated and are very resistant to further oxidation. The high potential of the yeast T. asahii SBUG-Y 833 to form different hydroxylated coumarines from alkylaromatics suggests possible applications in the biotechnological production of coumarine structures with medical potential as anticoagulative and antitumor pharmaceutical.



Similar content being viewed by others
References
Ammund OO, Higgins IJ (1985) The degradation of 1-phenylalkanes by an oil-degrading strain of Acinetobacter lwoffi. Antonie Van Leeuwenhoek 51:45–56
Awe S, Mikolasch A, Hammer E, Schauer F (2008) Degradation of phenylalkanes and characterization of aromatic intermediates acting as growth inhibiting substances in hydrocarbon utilizing yeast Candida maltosa. Int Biodeter Biodegr 62:408–414
Barnett JA, Payne RW, Yarrow D (2000) Yeasts, characteristics and identification, 3rd edn. Cambridge University Press, Cambridge, UK, p 752
Bellis DM, Spring MS, Stoker JR (1966) The biosynthesis of dicoumarol. Biochem J 103:202–206
Buswell JA (2001) Fungal biodegradation of chlorinated monoaromatics and BTEX compounds. In: Gadd GM (ed) Fungi in bioremediation. Cambridge University Press, Cambridge, UK, pp 113–135
Chagas-Neto TC, Chaves GM, Colombo AL (2008) Update on the genus Trichosporon. Mycopathol 166:121–132
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Davis JB, Raymond RL (1961) Oxidation of alkyl-substituted cyclic hydrocarbons by a Nocardia during growth on n-alkanes. Appl Microbiol 9:383–388
De Boer TD, Backer HJ (1956) Diazomethane. In: Leonard NJ (ed) Organic synthesis, vol 36. Wiley, New York, pp 14–16
Dutta TK (2005) Origin, occurrence, and biodegradation of long-side-chain alkyl compounds in the environment: a review. Environ Geochem Health 27:271–284
Dutta TK, Harayama S (2001a) Analysis of long-side chain alkylaromatics in crude oil for evaluation of their fate in the environment. Environ Sci Technol 35:102–107
Dutta TK, Harayama S (2001b) Biodegradation of n-alkylcycloalkanes and n-alkylbenzenes via new pathways in Alcanivorax sp. Strain MBIC 4326. Appl Environ Microbiol 67:1970–1974
Faber BW, van Gorcom RFM, Duine JA (2001) Purification and characterization of benzoate-para-hydroxylase, a cytochrome P450 (CYP53A1), from Aspergillus niger. Arch Biochem Biophys 394:245–254
Fedorak PM, Westlake DWS (1986) Fungal metabolism of n-alkylbenzenes. Appl Environ Microbiol 51:435–437
Fyfe D (ed) (1998) Oil Market Report, International Energy Agency. Available via http://omrpublic.iea.org/omrarchive/08dec98tab.pdf
Fyfe D (ed) (2008) Oil Market Report, International Energy Agency. Available via http://omrpublic.iea.org/omrarchive/11dec08tab.pdf
Guého E, Smith MT, de Hoog GS, Billon-Grand G, Christen R, der Vegte WH Batenburg-van (1992) Contributions to a revision of the genus Trichosporon. Antonie Van Leeuwenhoek 61:289–316
Guého E, Smith MT, de Hoog GS (1998) Trichosporon Behrend. In: Kurtzman CP, Fell JW (eds) The yeasts, a taxonomic study. Elsevier, Amsterdam, pp 854–872
Hornei S, Köhler M, Weide H (1972) Das Fettsäurespektrum eines Candida-Stammes nach Kultur auf n-Alkanen. Zeitschrift für Mikrobiologie 12:19–27
Johnson JS, Barry DA, Christofi N, Patel D (2001) Potential for anaerobic biodegradation of linear alkylbenzene cable oils: Literature review and preliminary investigation. Land Cont Reclam 9:279–291
Manolov I, Maichle-Moessmer C, Danchev N (2006) Synthesis, structure, toxicological and pharmacological investigations of 4-hydroxycoumarin derivatives. Eur J Med Chem 41:882–890
Matsuzaki F, Wariishi H (2005) Molecular characterization of cytochrome P450 catalyzing hydroxylation of benzoates from the white-rot fungus Phanaerochaete chrysosporium. Biochem Biophys Res Commun 334:1184–1190
Middelhoven WJ (1993) Catabolism of benzene compounds by ascomycetous and basidiomycetous yeasts and yeast-like fungi. Antonie Van Leeuwenhoek 63:125–144
Middelhoven WJ, Scorzetti G, Fell JW (2000) Trichosporon veenhuisii sp. nov., an alkane-assimilating anamorphic basidiomycetous yeast. Intern J System Evol Microbiol 50:381–387
Middelhoven WJ, Scorzetti G, Fell JW (2001) Trichosporon porosum comb. nov., an anamorphic basidiomycetous yeast inhibiting soil, related to the loubieri/laibachii group of species that assimilate hemicelluloses and phenolic compounds. FEMS Yeast Res 1:15–22
Middelhoven WJ (2003) Identification of clinically relevant Trichosporon species. Mycoses 46:7–11
Mingot JM, Peñalva MA, Fernández-Cañόn JM (1999) Disruption of phacA, an Aspergillus nidulans gene encoding a novel cytochrome P450 monooxygenase catalyzing phenylacetate 2-hydroxylation, results in penicillin overproduction. J Biol Chem 274(21):14545–14550
Penteado JC, Bruns RE, Franco de Carvalho LR (2006) Factorial design optimization of solid phase microextraction conditions for gas chromatography-mass spectrometry (GC-MS) analysis of linear alkylbenzenes (LABs) in detergents. Anal Chim Acta 562:152–157
Poulton JE, McRee DE, Conn EE (1980) Intracellular localization of two enzymes involved in coumarine biosynthesis in Melilotus alba. Plant Physiol 65:171–175
Rontani JF, Bertrand JC, Blanc F, Giusti G (1986) Gas chromatography and gas chromatography/mass spectrometry applied to the determination of a new pathway of pristane degradation by a marine mixed bacterial population. Mar Chem 18:9–16
Sariaslani FS, Harper DB, Higgins IJ (1974) Microbial degradation of hydrocarbons. Catabolism of 1-phenylalkanes by Nocardia salmonicolor. Biochem J 140:31–45
Schunck WH, Mauersberger S, Huth J, Riege P, Müller HG (1987) Function and regulation of cytochrome P-450 in alkane-asimilating yeast. I. Selective inhibition with carbon monoxide in growing cells. Arch Microbiol 147:240–244
Sietmann R, Hammer E, Moody J, Cerniglia CE, Schauer F (2000) Hydroxylation of biphenyl by the yeast Trichosporon mucoides. Arch Microbiol 174:353–361
Sietmann R, Hammer E, Schauer F (2002) Biotransformation of biarylic compounds by yeasts of the genus Trichosporon. System. Appl Microbiol 25:332–339
Smith MR, Ratledge C (1989) Catabolism of alkylbenzenes by Pseudomonas sp. NCIB 10643. Appl Microbiol Biotechnol 32:68–75
Spring MS, Stoker JR (1969) The biosynthesis of 4-hydroxycoumarin by Penicillium jenseni. Can J Biochem 47:301–304
Stoker JR (1964) The biosynthesis of coumarine in Melilotus alba. Biochem Biophys Res Commun 14(1):17–20
Van der Walt JP, Van Kerken AE (1961) The wine yeast of the cape. Part V. Studies on the occurrence of Brettanomyces intermedius and Brettanomyces schanderlii. Antonie van Leeuwenhoek 27:81–90
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Awe, S., Mikolasch, A. & Schauer, F. Formation of coumarines during the degradation of alkyl substituted aromatic oil components by the yeast Trichosporon asahii . Appl Microbiol Biotechnol 84, 965–976 (2009). https://doi.org/10.1007/s00253-009-2044-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2044-2