Abstract
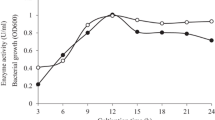
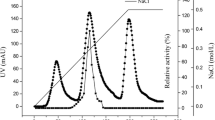
MAN5, the main extracellular saccharide hydrolase from Bacillus sp. MSJ-5, is an endo-β-mannanase with a demand of at least five sugar moieties for effective cleavage. It has a pH optimum of 5.5 and a temperature optimum of 50°C and is stable at pH 5–9 or below 65°C. MAN5 has a very high ability to hydrolyze konjac flour, 10 U/mg of which could completely liquefy konjac flour gum in 10 min at 50°C. HPLC analysis showed that most glucomannan in the konjac flour was hydrolyzed into a large amount of oligosaccharides with DP of 2–6 and a very small amount of monosaccharide. With the culture supernatant as enzyme source, the optimum condition to prepare oligosaccharides from konjac flour was obtained as 10 mg/ml konjac flour incubated with 10 U/mg enzyme at 50°C for 24 h. With this condition, more than 90% polysaccharides in the konjac flour solution were hydrolyzed into oligosaccharides and a little monosaccharide (2.98% of the oligosaccharides). Konjac flour is an underutilized agricultural material with low commercial value in China. With MAN5, konjac flour can be utilized to generate high value-added oligosaccharides. The high effectiveness and cheapness of this technique indicates its potential in industry.





Similar content being viewed by others
References
Araki T (1983) Purification and characterization of an endo-β-mannanase from Aeromonas sp F-25. J Fac Agric Kyushu Univ 27:89–98
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem 72:248–254
Cescutti P, Campa C, Delben F, Rizzo R (2002) Structure of the oligomers obtained by enzymatic hydrolysis of the glucomannan produced by the plant Amorphophallus konjac. Carbo Res 337:2505–2511
Daskiran M, Teeter RG, Fodge D, Hsiao HY (2004) An evaluation of endo-β-D-mannanase (Hemicell) effects on broiler performance and energy use in diets varying in β-mannan content. Poult Sci 83:662–668
Dhawan S, Kaur J (2007) Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol 27(4):197–216
Gomes J, Steiner W (1998) Production of a high activity of an extremely thermostable β-mannanase by the thermophilic eubacterium Rhodothermus marinus, grown on locust bean gum. Biotechnol Lett 20:729–733
Hägglund P (2002) Mannan-hydrolysis by hemicellulases. Lund University, Lund, pp 24–25
Hirayama M (2002) Novel physiological functions of oligosaccharides. Pure Appl Chem 74:1271–1279
Hossain MZ, Abe J, Hizukuri S (1996) Multiple forms of β-mannanase from Bacillus sp. KK01. Enz Microb Tech 18:95–98
Hrmova M, Burton RA, Lahnstein J, Fincher GB (2006) Hydrolysis of (1, 4)-β-D-mannans in barley (Hordeum vulgare L.) is mediated by the concerted action of (1, 4)-β-D-mannan endohydrolase and β-D-mannosidase. Biochem J 399:77–90
Hu ZY, Li Y (2007) Pseudidiomarina sediminum sp. nov., a marine bacterium isolated from coastal sediments of Luoyuan Bay in China. Int J Syst Evol Microbiol 57:2572–2577
Kobayashi Y, Echigen R, Mada M, Mutai M (1987) Effects of hydrolyzates of konjac mannan and soybean oligosaccharides on intestinal flora in man and rats. In: Mitsuoka T (ed) Intestinal flora and food factors. Gakkai Shuppan Centre, Tokyo, pp 79–97
Kurakake M, Komaki T (2001) Production of β-mannanase and β-mannosidase from Aspergillus awamori K4 and their properties. Curr Microbiol 42:377–380
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nat 227:680–685
Larsson AM, Anderson L, Xu B, Muñoz IG, Usón I, Janson JC, Stålbrand H, Ståhlberg J (2006) Three-dimensional crystal structure and enzymic characterization of β-mannanase Man5A from blue mussel Mytilus edulis. J Mol Biol 357:1500–1510
Le Nours J, Anderson L, Stoll D, Stålbrand H, Lo Leggio L (2006) The structure and characterization of a modular endo-beta-1, 4-mannanase from Cellulomonas fimi. Biochem 44:12700–12708
Lu XJ, Chen XM, Fu DX, Cong W, Ouyang F (2002) Effect of Amorphophallus konjac oligosaccharides on STZ-induced diabetes model of isolated islets. Life Sci 72:711–719
Mao SM, Zhang HY, Zhang XW (2007) Clone and expression of β-mannanase gene from Bacillus subtilis. J Agric Biotechnol 15:360–361
McCleary BV (1988) β-D-Mannanase. Methods Enzymol 160:596–610
Mendoza NS, Arai M, Kawaguchi T, Yoshida T, Joson LM (1994) Purification and properties of mannanase from Bacillus subtilis. World J Microbial Biotechnol 10:551–555
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Moreira LR, Filho EX (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Nakakuki T (2002) Present status and future of functional oligosaccharide development in Japan. Pure Appl Chem 74:1245–1251
Ootsuka S, Saga N, Suzuki K, Inoue A, Ojima T (2006) Isolation and cloning of an endo-β-1, 4-mannanase from Pacific abalone Haliotis discus hannai. J Biotechnol 125:269–280
Puchart V, Vršanská M, Svoboda P, Pohl J, Ögel ZB, Biely P (2004) Purification and characterization of two forms of endo-β-1, 4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749). Biochim Biophy Acta 1674:239–250
Reese ET, Shibata Y (1965) β-Mannanases of fungi. Can J Microbiol 11:167–183
Sako T, Matsumoto K, Tanaka R (1999) Recent progress on research and applications of non-digestible galacto-oligosaccharides. Int Dai J 9:69–80
Schröder R, Wegrzyn TF, Sharma NN, Atkinson RG (2006) LeMAN4 endo-β-mannanase from ripe tomato fruit can act as a mannan transglycosylase or hydrolase. Planta 224:1091–1102
Sheng DX, Teng JX (2008) Present status and future of konjac industry. China Agric Infor 7:39–40
Shimahara H, Suzuki H, Sugiyama N, Nisizawa K (1975) Partial purification of β-mannanase from the konjac tubers and their substrate specificity in relation to the structure of konjac glucomannan. Agric Biol Chem 39:301–312
Solange IM, Ismael MM (2007) Non-digestible oligosaccharides: a review. Carbohy Polymers 68:587–597
Tamaru Y, Araki T, Amagoi H, Mori H, Morishita T (1995) Purification and characterization of an extracellular β-1, 4-mannanase from a marine bacterium, Vibrio sp. strain MA-138. Appl Environ Microbiol 61:4454–4458
Wong KKY, Saddler JN (1993) Applications of hemicellulases in the food, feed, and pulp and paper industries. In: Coughlan MP, Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland, London, pp 127–143
Yamaura I, Nozaki Y, Matsumoto T, Kato T (1993) Purification and some properties of endo-1, 4-β-D-mannanase from a mud snail, Pomacea insularus (de Ordigny). Biosci Biotech Biochem 57:1316–1319
Yoon KH, Lim BL (2007) Cloning and strong expression of a Bacillus subtilis WL-3 mannanase gene in B. subtilis. J Microbiol Biotechnol 17:1688–1694
Zakaria MM, Yamamoto S, Yagi T (1998) Purification and characterization of an endo-1, 4-β-mannanase from Bacillus subtilis KU-1. FEMS Microbiol Lett 158:25–31
Zhang J, He ZM, Hu K (2000) Purification and characterization of β-mannanase from Bacillus licheniformis for industrial use. Biotechnol Lett 22:1375–1378
Acknowledgments
The work was supported by the Program for New Century Excellent Talents in University (NCET-04-0637), Foundation for Young Excellent Scientists in Shandong Province (2004BS06001), and COMRA Program (DYXM-115-02-2-6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Min Zhang and Xiu-Lan Chen contributed equally to this work.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 5718 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Chen, XL., Zhang, ZH. et al. Purification and functional characterization of endo-β-mannanase MAN5 and its application in oligosaccharide production from konjac flour. Appl Microbiol Biotechnol 83, 865–873 (2009). https://doi.org/10.1007/s00253-009-1920-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1920-0