Abstract
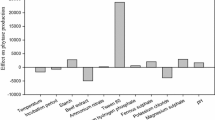
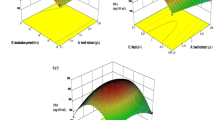
Medium composition and culture conditions for the bleaching stable alkaline protease production by Aspergillus clavatus ES1 were optimized. Two statistical methods were used. Plackett–Burman design was applied to find the key ingredients and conditions for the best yield. Response surface methodology (RSM) including full factorial design was used to determine the optimal concentrations and conditions. Results indicated that Mirabilis jalapa tubers powder (MJTP), culture temperature, and initial medium pH had significant effects on the production. Under the proposed optimized conditions, the protease experimental yield (770.66 U/ml) closely matched the yield predicted by the statistical model (749.94 U/ml) with R 2 = 0.98. The optimum operating conditions obtained from the RSM were MJTP concentration of 10 g/l, pH 8.0, and temperature of 30 °C, Sardinella heads and viscera flour (SHVF) and other salts were used at low level. The medium optimization contributed an about 14.0-fold higher yield than that of the unoptimized medium (starch 5 g/l, yeast extract 2 g/l, temperature 30 °C, and pH 6.0; 56 U/ml). More interestingly, the optimization was carried out with the by-product sources, which may result in cost–effective production of alkaline protease by the strain.


Similar content being viewed by others
References
Adinarayana K, Ellaiah P, Srinivasulu B, Bhavani Devi R, Adinarayana G (2003) Response surface methodological approach to optimize the nutritional parameters for neomycin production by Streptomyces marinensis under solid-state fermentation. Process Biochem 38:1565–1572
Agrawal D, Patidar P, Banerjee T, Patil S (2005) Alkaline protease production by a soil isolate of Beauveria felina under SSF condition: parameter optimization and application to soy protein hydrolysis. Process Biochem 40:1131–1136
Banerjee UC, Sani RK, Azmi W, Soni R (1999) Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem 35:213–219
Calik P, Özdamar TH (2001) Carbon sources affect metabolic capacities of Bacillus species for the production of industrial enzymes: theoretical analyses for serine and neutral proteases and a-amylase. Biochem Eng J 8:61–81
Chakraborty R, Srinivasan M, Sarkar SK, Raghavan KV (1995) Production of acid protease by a new Aspergillus niger by solid substrate fermentation. J Microb Biotechnol 10:17–30
Chi Z, Zhao S (2003) Optimization of medium and cultivation conditions for pullulan production by a new pullulan-producing yeast strain. Enzyme Microb Technol 33:206–211
Chu IM, Lee C, Li TS (1992) Production and degradation of alkaline protease in batch cultures of Bacillus subtilis ATCC 14416. Enzyme Microb Technol 14:755–761
de Coninck J, Bouquelet S, Dumortier V, Duyme F, Verdier-Denantes I (2000) Industrial media and fermentation processes for improved growth and protease production by Tetrahymena thermophila. J Ind Microbiol Biotech 24:285–290
Dutta JR, Dutta PK, Banerjee R (2004) Optimization of culture parameters of extracellular protease production from a newly isolated Pseudomonas sp. using response surface and artificial neural network models. Process Biochem 39:2193–2198
Ellouz Y, Bayouth A, Kammoun S, Gharsallah N, Nasri M (2001) Production of protease by Bacillus subtilis grown on sardinelle heads and viscera flour. Bioresour Technol 80:49–51
Germano S, Pandey A, Osaku CA, Rocha SN, Soccol CR (2003) Characterization and stability of proteases from Penicillium sp. produced by solid-state fermentation. Enzyme Microb Technol 32:246–251
Gupta R, Beg QK, Lorenz P (2002a) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Gupta R, Beg QK, Khan S, Chauhan B (2002b) An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biotechnol 60:381–395
Haaland PD (1989) Statistical problem solving. In: Haaland PD (ed) Experimental design in biotechnology. Dekker, New York, pp 1–18
Hajji M, Kanoun S, Nasri M, Gharsallah N (2007) Purification and characterization of an alkaline serine-protease produced by a new isolated Aspergillus clavatus ES1. Process Biochem 42:791–797
Hanlon GW, Hodges NA, Russel AD (1982) The influence of glucose, ammonium and magnesium availability on the production of protease and bacitracin by Bacillus licheniformis. J Gen Microbiol 128:845–851
Hintz WE, Kalsner I, Plawinski E, Guo Z, Lagosky PA (1995) Improved gene expression in Aspergillus nidulans. Can J Bot 73:876–884
Hoffman B, Breuil C (2002) Cloning and genetic analysis of subtilases in sap staining fungi. Curr Genet 41:168–175
Johnvesly B, Naik GR (2001) Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochem 37:139–144
Kembhavi AA, Kulkarni A, Pant AA (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No 64. Appl Biochem Biotechnol 38:83–92
Kirk O, Borchert TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13:345–351
Kole MM, Draper I, Gerson DF (1988) Production of protease by Bacillus subtilis using simultaneous control of glucose and ammonium concentrations. J Chem Technol Biotechnol 41:197–206
Kumar CG, Takagi H (1999) Microbial alkaline proteases from a bioindustrial viewpoint. Biotechnol Adv 17:561–594
Kunamneni A, Kumar KS, Singh S (2005) Response surface methodology approach to optimize the nutritional parameters for enhance a-amylase. Afr J Biotechnol 4:708–716
Laxman RS, Sonawane AP, More SV, Rao BS, Rele MV, Jogdand VV, Deshpande VV, Rao MB (2005) Optimization and scale up of production of alkaline protease from Conidiobolus coronatus. Process Biochem 40:3152–3158
Malathi S, Chakraborty R (1991) Production of alkaline protease by a new Aspergillus flavus isolate under solid-substrate fermentation conditions for use as a depilation agent. Appl Environ Microbiol 57:712–716
Mitchell DA, Lonsane BK (1992) Definition, characteristics and potential in solid state cultivation. In: Doelle HW, Mitchell SA, Rolz CE (eds) Applied biotechnology series. Elsevier, Amsterdam, pp 1–16
Nehete PN, Shah VD, Kothari RM (1985) Profiles of alkaline protease production as a function of composition of the slant, age, transfer and isolate number and physiological state of culture. Biotechnol Lett 7:413–418
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Santos RMDB, Firmino AAP, de Sá CM, Felix CR (1996) Keratinolytic activity of Aspergillus fumigatus Fresenius. Curr Microbiol 33:364–370
Varela H, Ferrari MD, Belobradjic L, Weyrauch R, Loperena L (1996) Effect of medium composition on the production by a new Bacillus subtilis isolate of protease with promising unhairing activity. World J Microbiol Biotechnol 12:643–645
Wang SL, Chen YH, Wang CL, Yen YH, Chern MK (2005) Purification and characterization of a serine protease extracellularly produced by Aspergillus fumigatus in a shrimp and crab shell powder medium. Enzyme Microb Technol 36:660–665
Wang Q, Hou Y, Xu Z, Miao J, Li G (2007) Optimization of cold-active protease production by the psychrophilic bacterium Colwellia sp. NJ341 with response surface methodology. Bioresour Technol 99:1926–1931
Weisberg S (1985) Applied linear regression. Wiley, New York, p 217
Acknowledgments
We are grateful to Mme Hanen Ghamgui and Mr. Fakher Frikha for their help in the RSM software analysis. We also thank Mr. Zouhaier Bouallagui and Mme Jaouher Zekri for the help with the English writings. This work was financed by the “Ministere de l’enseignement supérieur, de la recherche scientifique et de la technologie, Tunisia”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajji, M., Rebai, A., Gharsallah, N. et al. Optimization of alkaline protease production by Aspergillus clavatus ES1 in Mirabilis jalapa tuber powder using statistical experimental design. Appl Microbiol Biotechnol 79, 915–923 (2008). https://doi.org/10.1007/s00253-008-1508-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1508-0