Abstract
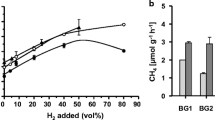
The importance of syntrophic relationships among microorganisms participating in biogas formation has been emphasized, and the regulatory role of in situ hydrogen production has been recognized. It was assumed that the availability of hydrogen may be a limiting factor for hydrogenotrophic methanogens. This hypothesis was tested under laboratory and field conditions by adding a mesophilic (Enterobacter cloacae) or thermophilic hydrogen-producing (Caldicellulosyruptor saccharolyticus) strain to natural biogas-producing consortia. The substrates were waste water sludge, dried plant biomass from Jerusalem artichoke, and pig manure. In all cases, a significant intensification of biogas production was observed. The composition of the generated biogas did not noticeably change. In addition to being a good hydrogen producer, C. saccharolyticus has cellulolytic activity; hence, it is particularly suitable when cellulose-containing biomass is fermented. The process was tested in a 5-m3 thermophilic biogas digester using pig manure slurry as a substrate. Biogas formation increased at least 160–170% upon addition of the hydrogen-producing bacteria as compared to the biogas production of the spontaneously formed microbial consortium. Using the hydrogenase-minus control strain provided evidence that the observed enhancement was due to interspecies hydrogen transfer. The on-going presence of C. saccharolyticus was demonstrated after several months of semicontinuous operation.






Similar content being viewed by others
References
Achnich C, Bak F, Conrad R (1995a) Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers and methanogens in anoxic paddy soil. Biol Fertil Soils 19:65–72
Achnich C, Schuhmann A, Wind T, Conrad R (1995b) Role of interspecies H2 transfer to sulfate and ferric iron-reducing bacteria in acetate consumption in anoxic paddy soil. FEMS Microbiol Ecol 16:61–70
Ahring BK (2003) Perspectives for anaerobic digestion. Adv Biochem Eng Biotechnol 81:1–30
Ahring BK, Westermann P (1988) Product inhibition of butyrate metabolism by acetate and hydrogen in a thermophilic coculture. Appl Environ Microbiol 54:2393–2397
Ahring BK, Westermann P, Mah RA (1991) Hydrogen inhibition of acetate metabolism and kinetics of hydrogen consumption by Methanosarcina thermophila TM-1. Arch Microbiol 157:38–42
Bagi Z, Perei K, Rákhely G, Kovács KL (2004) Biotechnological procedure for the intensification of biogas production in a thermophilic system. Hungarian Patent no. P0402444/2004
Bernstead J, Archer DB, Lloyd D (1990) Role of hydrogen in the growth of mutualistic methanogenic cocultures. In: Bélaich J-P, Bruschi M, Garcia J-L (eds) Microbiology and biochemistry of strict anaerobes involved in interspecies transfer. Plenum, New York, pp 161–171
Boone DR, Bryant MP (1980) Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov. from methanogenic ecosystems. Appl Environ Microbiol 40:626–632
Bryant MP, Wolin EA, Wolin MJ, Wolfe RS (1967) Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Microbiol 59:20–31
Cayol J-L, Fardeau M-L, Garcia J-L, Ollivier B (2002) Evidence of interspecies hydrogen transfer from glycerol in saline environments. Extremophiles 6:131–134
Conrad R, Bonjour F, Aragno M (1985a) Aerobic and anaerobic microbial consumption of hydrogen in geothermal spring water. FEMS Microbiol Lett 29:201–205
Conrad R, Phelps TJ, Zeikus JG (1985b) Gas metabolism evidence in support of juxtaposition of hydrogen-producing and methanogenic bacteria in sewage sludge and lake sediments. Appl Environ Microbiol 50:595–601
Conrad R, Lupton FS, Zeikus JG (1987) Hydrogen metabolism and sulfate-dependent inhibition of methanogenesis in eutrophic lake sediment (Lake Mendota). FEMS Microbiol Ecol 45:107–115
Das D, Veziroglu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Energy 26:13–28
Dolfing J (1985) Kinetics of methane formation by granular sludge at low substrate concentrations, the influence of mass transfer limitation. Appl Microbiol Biotechnol 22:77–81
Dolfing J, Blomen WGBM (1985) Activity measurements as a tool to characterize the microbial composition of methanogenic environments. J Microbiol Methods 4:1–12
Fernandez AS, Hashsham SA, Dollhope SL, Raskin L, Glagoleva O, Dazzo FB, Hickey RF, Criddle CS, Tiedje JM (2000) Flexible community structure correlates with stable community function in methanogenic bioreactor communities perturbed by glucose. Appl Environ Microbiol 66:4058–4067
Gray NF (2004) Biology of wastewater treatment (2nd edn.). Imperial College, London
Guyer W, Zehnder AJB (1982) Conversion processes in anaerobic digestion. Water Sci Technol 15:1457–1461
Hallenbeck PC, Benemann JR (2002) Biological hydrogen production: fundamentals and limiting processes. Int J Hydrogen Energy 27:1185–1193
Hashsham SA, Fernandez AS, Dollhope SL, Dazzo FB, Hickley RF, Tiedje JM, Criddle CS (2000) Parallel processing of substrate correlates with greater functional stability in methanogenic bioreactor communities perturbed by glucose. Appl Environ Microbiol 66:4050–4057
Hofman-Bang J, Zheng D, Westermann P, Ahring BK, Raskin L (2003) Molecular ecology of anaerobic reactor systems. Adv Biochem Eng Biotechnol 81:153–203
Kovács KL, Polyák B (1991) Hydrogenase reactions and utilization of hydrogen in biogas production and microbiological denitrification systems. In: Proc. 4th IGT Symp. on Gas, Oil, and Environmental Biotechnology, Chapter 5, Colorado Springs, pp 1–16
Kovács KL, Bagyinka C, Verebély I (1985) Procedure for the augmentation of biogas production in anaerobic fermentation using mixed bacterial populations. Hungarian Patent no. 195 978
Kovács KL, Kovács ÁT, Maróti G, Bagi Z, Csanádi G, Perei K, Bálint B, Balogh J, Fülöp A, Mészáros LS, Tóth A, Dávid R, Latinovics D, Varga A, Rákhely G (2004) Improvement of biohydrogen production and intensification of biogas formation. Rev Environ Sci Biotechnol 3(3):321–330
Krylova NI, Janssen PH, Conrad R (1997) Turnover of propionate in methanogenic paddy soil. FEMS Microbiol Ecol 23:107–117
Lettinga G, Haandel AC (1993) Anaerobic digestion for energy production and environmental protection. In: Johanson TB (ed) Renewable energy: sources for fuels and electricity. Island Press, California, pp 817–839
Lovley DR, Dwyer DF, Klug MJ (1982) Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol 43:1373–1379
Magalon A, Böck A (2000) Analysis of the HypC–HycE complex, a key intermediate in the assembly of the metal center of the Escherichia coli hydrogenase 3. J Biol Chem 275:21114–21120
Miron YG, Zeeman G, van Lier JB, Lettinga G (2000) The role of sludge retention time in the hydrolysis and acidification of lipids, carbohydrates and proteins during digestion of primary sludge in CSTR systems. Water Res 34(5):1705–1713
Pind PF, Angelidaki I, Ahring BK (2003) Dynamics of the anaerobic process: effects of volatile fatty acids. Biotechnol Bioeng 82(7):791–801
Rainey FA, Donnison AM, Janssen PH, Saul D, Rodrigo A, Bergquist PL, Daniel RM, Stackebrandt E, Morgan HW (1994) Description of Caldicellulosiruptor saccharolyticus gen. nov., sp. nov.: an obligately anaerobic, extremely thermophilic, cellulolytic bacterium. FEMS Microbiol Lett 120:263–266
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn.). Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Schmidt JE, Ahring BK (1993) Effects of hydrogen and formate on the degradation of propionate and butyrate in thermophilic granules from an upflow anaerobic sludge blanket reactor. Appl Environ Microbiol 59:2546–2551
Thiele JH, Zeikus JG (1988a) Control of interspecies electron flow during anaerobic digestion: significance of formate transfer versus hydrogen transfer during syntrophic methanogenesis in flocs. Appl Environ Microbiol 54:20–29
Thiele JH, Zeikus JG (1988b) Interactions between hydrogen- and formate-producing bacteria and methanogens during anaerobic digestion. In: Erickson LE, Fung DY (eds) Handbook on anaerobic fermentation. Marcel Dekker, New York, pp 537–547
Thiele JH, Chartrain M, Zeikus JG (1988) Control of interspecies electron flow during anaerobic digestion: role of floc formation in syntrophic methanogenesis. Appl Environ Microbiol 54:10–19
van Niel EWJ, Budde MAW, de Haas GG, van der Wal FJ, Claassen PAM, Stams AJM (2002) Distinctive properties of high hydrogen producing extreme thermophiles, Caldicellulosyruptor saccharolyticus and Thermotoge elfi. Int J Hydrogen Energy 27:1391–1398
van Niel EW, Claassen PAM, Stams AJM (2003) Substrate and product inhibition of hydrogen production by the extreme hyperthermophile, Caldicellulosyruptor saccharolyticus. Biotechnol Bioeng 81:255–257
Wolin MJ (1975) Interspecies hydrogen transfer between H2-producing and methane-producing species. In: Schlegel HG, Gottschalk G, Pfenning N (eds) Symposium on microbial production and utilization of gases. Akademie der Wissenschaften, Göttingen, Germany, pp 141–150
Wu W-M, Hickey RF, Zeikus JG (1991) Characterization of metabolic performance of methanogenic granules treating brewery wastewater: role of sulfate-reducing bacteria. Appl Environ Microbiol 57:3438–3449
Zeeman G, Lettinga G (1999) The role of anaerobic digestion of domestic sewage in closing the water and nutrient cycle at community level. Water Sci Technol 39(5):187–194
Acknowledgment
This work has been partly supported by EU 6th Framework Programme projects (HyVolution SES6 019825 and NEST STRP SOLAR-H 5166510), by EBARA (Japan), and by domestic funds (NKTH, GVOP, Asbóth, Baross, DEAK-KKK, KN-RET). International collaboration within the EU network COST Action 841 and AD-NETT-1 and AD-NETT-2 is greatly appreciated. We thank Prof. Dr. August Böck and Dr. Axel Magalon (Ludwig–Maximilian University, Münich, Germany) for the hypF mutant E. coli strain. The authors also thank Dr. Csaba Bagyinka who took part in the early phase of the mesophilic experiments and Dr. Pál Valastyán and Mr. Gergely Kovács for their assistance in the thermophilic scale-up experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagi, Z., Ács, N., Bálint, B. et al. Biotechnological intensification of biogas production. Appl Microbiol Biotechnol 76, 473–482 (2007). https://doi.org/10.1007/s00253-007-1009-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1009-6