Abstract
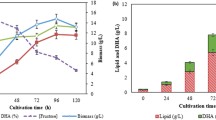
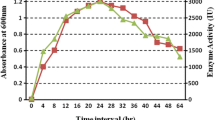
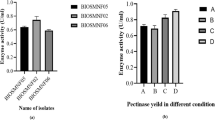
In this work, a 22 factorial design was employed combining with response surface methodology (RSM) to optimize the medium compositions for the production of alkaline β-mannanase by alkaliphilic Bacillus sp. N16-5 isolated previously from sediment of Wudunur Soda Lake in Inner Mongolia, China. The central composite design (CCD) used for the analysis of treatment combinations showed that a second-order polynomial regression model was in good agreement with experimental results, with R 2 = 0.9829 (P < 0.05). The maximum activity was obtained at NaCl concentration (84.4 g l−1) and sodium glutamate (3.11 g l−1) and a high medium pH around 10.0. Under such conditions, the activity of alkaline β-mannanase achieved 310.1 U/ml in the scale of 5-l fermenter, which was increased nearly twice compared with the original. Through optimization, the substrates shifted from the expensive substrates, such as locust bean gum and peptone, to the inexpensive ones such as konjac powder, soymeal, and sodium glutamate. The experiment results also suggested that the environmental conditions of high salinity and high alkalinity, as well as the inducer substrates, play very important roles in the production of the alkaline β-mannanase by alkaliphilic Bacillus sp. N16-5.



Similar content being viewed by others
Reference
Adams MWW, Perter FB, Kelly RM (1995) Extremozymes: expanding the limits of biocatalysis. Biotechnol 13:662–668
Akino T, Nakamura N, Horikoshi K (1987) Production of β-mannosidase and β-mannanase by an alkalophilic Bacillus sp. Appl Microbiol Biotechnol 26:323–327
Akino T, Kato C, Horikoshi K (1989) The cloned β-mannanase gene from alkalophilic Bacillus sp. AM-001 produces two β-mannanases in Escherichia coli. Arch Microbiol 152:10–15
Bettiol JP, Showell MS (2002) Detergent compositions comprising a mannanase and a protease. US Patent 6,376,445
Clarke JH, Davidson K, Rixon JE, Halstead JR, Fransen MP, Gilbert HJ, Hazlewood GP (2000) A comparison of enzyme-aided bleaching of softwood paper pulp using combinations of xylanase, mannanase and á-galactosidase. Appl Microbiol Biotechnol 53:661–667
Cui FJ, Li Y, Xu ZH, Xu HY, Sun K, Tao WY (2006) Optimization of the medium composition for production of mycelial biomass and exo-polymer by Grifola frondosa GF9801 using response surface methodology. Bioresour Technol 97:1209–1216
Detkova EN, Pusheva MA (2006) Energy metabolism in halophilic and alkaliphilic acetogenic bacteria. Microbiology 75:1–11
Gupta S, Bhushan B, Hoondal GS (1999) Enhanced production of xylanase from Staphylococcus sp. SG-13 using amino acids. World J Microbiol Biotechnol 15:511–512
Hatada Y, Takeda N, Hirasawa K, Ohta Y, Usami R, Yoshida Y, Grant WD, Ito S, Horikoshi K (2005) Sequence of the gene for a high-alkaline mannanase from an alkaliphilic Bacillus sp. strain JAMB-750, its expression in Bacillus subtilis and characterization of the recombinant enzyme. Extremophiles 9:497–500
Heck JX, De Barros Soares LH, Ayub MAZ (2005a) Optimization of xylanase and mannanase production by Bacillus circulans strain BL53 on solid-state cultivation. Enzyme and Microb Technol 37:417–423
Heck JX, Flores SH, Hertzm PF, Ayub MAZ (2005b) Optimization of cellulase-free xylanase activity produced by Bacillus coagulans BL69 in solid-state cultivation. Process Biochem 40:107–112
Horikoshi K (1971) Production of alkaline enzymes by alkalophilic microorganisms. Part I. Alkaline protease produced by Bacillus No. 221. Agric Biol Chem 36:1407–1414
Hossain MZ, Abe J, Hizukuri S (1996) Multiple forms of β-mannanase from Bacillus sp. KK0l. Enzyme and Microb Technol 18:95–98
Ikura Y, Horikoshi K (1987) Stimulatory effect of certain amino acids on xylanase production by alkalophilic Bacillus sp. Agric Biol Chem 51:3143–3145
Kalil SJ, Maugeri F, Rodrigues MI (2000) Response surface analysis and simulation as a tool for bioprocess design and optimization. Process Biochem 35:539–550
Kansoh AL, Nagieb ZA (2004) Xylanase and mannanase enzymes from Streptomyces galbus NR and their use in biobleaching of softwood kraft pulp. Antonie van Leeuwenhoek 85:103–114
Levin L, Forschiassin F (1998) Influence of growth conditions on the production of xylanolytic enzymes by Trametes trogii. World J Microbiol Biotechnol 14:443–446
Liu JZ, Weng LP, Zhang QL, Xu H, Ji LN (2003) Optimization of glucose oxidase production by Aspergillus niger in a benchtop bioreactor using response surface methodology. World J Microbiol Biotechnol 19:317–323
Ma YH (1999) Alkaliphiles. Microbiology 26:309 (in Chinese)
Ma YH, Tian XY, Zhou PJ, Wang DZ (1991) Production and some properties of alkaline β-mannanase. Acta Microbiol Sin 31:443–448 (in Chinese)
Ma YH, Xue YF, Dou YT, Xu ZH, Tao WY, Zhou PJ (2004) Characterization and gene cloning of a novel β-mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles 8:447–454
McCleary BV (1988) β-D-Mannanase. Methods Enzymol 160:596–610
McCutchen CM, Duffaud GD, Leduc P, Petersen ARH, Tayal A, Khan SA, Kelly RM (1996) Characterization of extremely thermostable enzymatic breakers (α-1,6-galactosidase and β-1,4-mannanase) from the hyperthermophilic bacterium Thermotoga meapolitana 5068 for hydrolysis of guar gum. Biotechnol Bioeng 52:332–339
Oh S, Rheem S, Sim J, Kim S, Back Y (1995) Optimizing conditions for the growth of Lactobacillus casei YIT9018 in tyrptone-yeast extract-glucose medium by using response surface methodology. Appl Environ Microbiol 61:3809–3814
Plackett RL, Burman JP (1946) The design of optimum multi-factorial experiments. Biometrika 33:305–325
Schiradi C, De Rose M (2002) The production of biocatalysts and biomolecules from extremophiles. Trends Biotechnol 20:515–521
Wang YX, Lv FX, Lu ZX (2004) Optimization of cultivation medium Clitocybe sp.AS5.112 for the extracellular polysaccharide production and mycelial growth by response surface methodology. Journal of NanJing Agriculture University 27:89–94
Acknowledgment
This work was supported by grants of National Basic Research Program of China (No.2007CB707804), National High-Tech Program (No. 2006AA020104), Jiangsu Planned Projects for Postdoctoral Research Funds (No.0601004A), and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT0532). The authors would thank Dr. Luhong Tang for his critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, Ss., Dou, Wf., Xu, Hy. et al. Optimization of medium composition for the production of alkaline β-mannanase by alkaliphilic Bacillus sp. N16-5 using response surface methodology. Appl Microbiol Biotechnol 75, 1015–1022 (2007). https://doi.org/10.1007/s00253-007-0907-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0907-y