Abstract
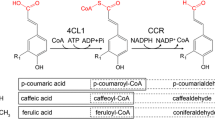
For the fermentative production of plant-specific flavanones (naringenin, pinocembrin) by Escherichia coli, a plasmid was constructed which carried an artificial biosynthetic gene cluster, including PAL encoding a phenylalanine ammonia-lyase from a yeast, ScCCL encoding a cinnamate/coumarate:CoA ligase from the actinomycete Streptomyces coelicolor A3(2), CHS encoding a chalcone synthase from a licorice plant and CHI encoding a chalcone isomerase from the Pueraria plant. The recombinant E. coli cells produced (2S)-naringenin from tyrosine and (2S)-pinocembrin from phenylalanine. When the two subunit genes of acetyl-CoA carboxylase from Corynebacterium glutamicum were expressed under the control of the T7 promoter and the ribosome-binding sequence in the recombinant E. coli cells, the flavanone yields were greatly increased, probably because enhanced expression of acetyl-CoA carboxylase increased a pool of malonyl-CoA that was available for flavanone synthesis. Under cultural conditions where E. coli at a cell density of 50 g/l was incubated in the presence of 3 mM tyrosine or phenylalanine, the yields of naringenin and pinocembrin reached about 60 mg/l. The fermentative production of flavanones in E. coli is the first step in the construction of a library of flavonoid compounds and un-natural flavonoids in bacteria.





Similar content being viewed by others
References
Bednar RA, Hadcock JR (1988) Purification and characterization of chalcone isomerase from soybeans. J Biol Chem 263:9582–9588
Bradley JM, Deroles SC, Boase MR, Bloor S, Swinny E, Davis KM (1999) Variation in the ability of the maize Lc regulatory gene to upregulate flavonoid biosynthesis in heterologous systems. Plant Sci 140:31–39
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56:317–333
Davis MS, Cronan JE Jr (2001) Inhibition of Escherichia coli acetyl-CoA carboxylase by acyl–acyl carrier protein. J Bacteriol 183:1499–1503
Davis MS, Solbiati J, Cronan JE Jr (2000) Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem 275:28593–28598
Dixon RA, Steele CL (1999) Flavonoids and isoflavonoids—a gold mine for metabolic engineering. Trends Plant Sci 4:394–400
File SE, Jarrett N, Fluck E, Duffy R, Casey K, Wiseman H (2001) Eating soya improves human memory. Psychopharmacology 157:430–436
Grotewold E, Chamberlin M, Snok M, Siame B, Butler L, Swenson J, Maddock S, St Clair G, Browen B (1998) Engineering secondary metabolism in maize cells by ectopic expression of transcription factors. Plant Cell 10:721–740
Hotze M, Schröder G, Schröder J (1995) Cinnamate 4-hydroxylase from Catharanthus roseus, and a strategy for the functional expression of plant cytochrome P450 proteins as transcriptional fusions with P450 reductase in E. coli. FEBS Lett 374:345–350
Hwang EI, Kaneko M, Ohnishi Y, Horinouchi S (2003) Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster. Appl Environ Microbiol 69:2699–2706
Jez JM, Bowman ME, Dixon RA, Noel JP (2000) Structure and mechanism of evolutionarily unique enzyme chalcone isomerase. Nat Struct Biol 7:786–791
Kaneko M, Hwang EI, Ohnishi Y, Horinouchi S (2003a) Heterologous production of flavanones in Escherichia coli: potential for combinatorial biosynthesis of flavonoids in bacteria. J Ind Microbiol Biotechnol 30:456–461
Kaneko M, Ohnishi Y, Horinouchi S (2003b) Cinnamate: coenzyme A ligase from the filamentous bacterium Streptomyces coelicolor A3(2). J Bacteriol 185:20–27
Krause M, Galensa R (1991) Analysis of enantiomeric flavanones in plant extracts by high-performance liquid chromatography on a cellulose triacetate based chiral stationary phase. Chromatographia 32:69–72
Kyndt JA, Meyer TE, Cusanovich MA, Van Beeumen JJ (2002) Characterization of a bacterial tyrosine ammonia lyase, a biosynthetic enzyme for the photoactive yellow protein. FEBS Lett 512:240–244
Lamartiniere CA (2000) Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr 71:1705S–1707S
Le Marchand L (2002) Cancer preventive effects of flavonoids—a review. Biomed Pharmacother 56:296–301
Mol JNM, Robbinst MP, Dixon RA, Veltkamp E (1985) Spontaneous and enzymic rearrangement of naringenin chalcone to flavanone. Phytochemistry 24:2267–2269
Pohl NL (2002) Nonnatural substrates for polyketide synthases and their associated modifying enzymes. Curr Opin Chem Biol 6:773–778
Rodriguez E, McDaniel R (2001) Combinatorial biosynthesis of antimicrobials and other natural products. Curr Opin Microbiol 4:526–534
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
Schröder J (1997) A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci 2:373–378
Scott DA, Hammond PM, Brearly GM, Price CP (1992) Identification by high-performance liquid chromatography of tyrosine ammonia-lyase activity in purified fractions of Phaseolus vulgaris phenylalanine ammonia-lyase. J Chromatogr 575:309–312
Takamura Y, Nomura G (1988) Changes in the intracellular concentration of acetyl-CoA and malonyl-CoA in relation to the carbon and energy metabolism of Escherichia coli K12. J Gen Microbiol 134:2249–2253
Terai Y, Fujii T, Byun S-H, Nakajima O, Hakamatsuka T, Ebizuka Y, Sankawa U (1996) Cloning of chalcone–flavanone isomerase cDNA from Pueraria lobata and its overexpression in Escherichia coli. Protein Expr Purif 8:183–190
Watts KT, Lee PC, Schmidt-Dannert C (2004) Exploring recombinant flavonoid biosynthesis in metabolically engineered Escherichia coli. Chem Biochem 5:500–507
Weisshaar B, Jenkins GI (1998) Phenylpropanoid biosynthesis and its regulation. Curr Opin Plant Biol 1:251–257
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Acknowledgements
This work was supported, in part, by the Bio Design Program of the Ministry of Agriculture, Forestry, and Fisheries of Japan and by a grant from the Industrial Technology Research Grant Program 2000 of the New Energy and Industrial Technology Development Organization of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyahisa, I., Kaneko, M., Funa, N. et al. Efficient production of (2S)-flavanones by Escherichia coli containing an artificial biosynthetic gene cluster. Appl Microbiol Biotechnol 68, 498–504 (2005). https://doi.org/10.1007/s00253-005-1916-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-1916-3