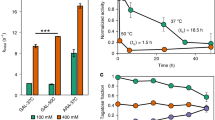
Abstract
The ability to convert d-galactose into d-tagatose was compared among a number of bacterial l-arabinose isomerases (araA). One of the most efficient enzymes, from the anaerobic thermophilic bacterium Thermoanaerobacter mathranii, was produced heterologously in Escherichia coli and characterised. Amino acid sequence comparisons indicated that this enzyme is only distantly related to the group of previously known araA sequences in which the sequence similarity is evident. The substrate specificity and the Michaelis–Menten constants of the enzyme determined with l-arabinose, d-galactose and d-fucose also indicated that this enzyme is an unusual, versatile l-arabinose isomerase which is able to isomerise structurally related sugars. The enzyme was immobilised and used for production of d-tagatose at 65 °C. Starting from a 30% solution of d-galactose, the yield of d-tagatose was 42% and no sugars other than d-tagatose and d-galactose were detected. Direct conversion of lactose to d-tagatose in a single reactor was demonstrated using a thermostable β-galactosidase together with the thermostable l-arabinose isomerase. The two enzymes were also successfully combined with a commercially available glucose isomerase for conversion of lactose into a sweetening mixture comprising lactose, glucose, galactose, fructose and tagatose.



Similar content being viewed by others
References
Barkholt V, Jensen AL (1989) Amino acid analysis: determination of cysteine plus half-cystine in proteins after hydrochloric acid hydrolysis with a disulfide compound as additive. Anal Biochem 177:318–322
Beadle JR, Saunders JP, Wajda TJ (1991) US Patent 5002612
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cheetham PSJ, Wootton AN (1993) Bioconversion of d-galactose into d-tagatose. Enzyme Microb Technol 15:105–108
Childers SE, Vargas M, Noll KM (1992) Improved methods for cultivation of the extremely thermophilic bacterium Thermotoga neapolitana. Appl Env Microbiol 58:3949–3953
Dische Z, Borenfreund E (1951) A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem 192:583–587
Freimund S, Huwig A, Giffhorn F, Köpper S (1996) Convenient chemo-enzymatic synthesis of d-tagatose. J Carbohydr Chem 15:115–120
Haltrich D, Leitner C, Neuhauser W, Nidetzky B, Kulbe KD, Volc J (1998) A convenient enzymatic procedure for the production of aldose-free d-tagatose. Ann NY Acad Sci 864:295–299
Heath EC, Horecker BL, Smyrniotis PZ, Takagi Y (1958) Pentose fermentation by Lactobacillus plantarum II. l-Arabinose isomerase. J Biol Chem 231:1031–1037
Ibrahim OO, Spradlin JE (2000) US Patent 6057135
Izumori K, Tsuzaki K (1988) Production of d-tagatose from d-galactitol by Mycobacterium smegmatis. J Ferment Technol 66:225–227
Izumori K, Ueda Y, Yamanaka K (1978) Pentose metabolism in Mycobacterium smegmatis: comparison of l-arabinose isomerases induced by l-arabinose and d-galactose. J Bacteriol 133:413–414
Kim B-C, Lee Y-H, Lee H-S, Lee D-W, Choe E-A, Pyun Y-R (2002) Cloning, expression and characterization of l-arabinose isomerase from Thermotoga neapolitana: bioconversion of d-galactose to d-tagatose using the enzyme. FEMS Microbiol Lett 212:121–126
Kim J-W, Kim Y-W, Roh H-J, Kim H-Y, Cha J-H, Park K-H, Park C-S (2003) Production of tagatose by a recombinant thermostable l-arabinose isomerase from Thermus sp. IM6501. Biotechnol Lett 25:963–967
LeGendre N, Mansfield M, Weiss A, Matsudaira P (1993) Purification of proteins and peptides by SDS-PAGE. In: Matsudaira P (ed) A practical guide to protein and peptide purification for microsequencing, 2nd edn. Academic Press, San Diego, pp 74–101
Levin GV (2002) Tagatose, the new GRAS sweetener and health product. J Med Food 5:23–36
Manzoni M, Rollini M, Bergomi S (2001) Biotransformation of d-galactitol to tagatose by acetic acid bacteria. Process Biochem 36:971–977
McCammon SA, Innes BH, Bowman JP, Franzmann PD, Dobson SJ, Holloway PE, Skerratt JH, Nichols PD, Rankin LM (1998) Flavobacterium hibernum sp. nov., a lactose-utilizing bacterium from a freshwater Antarctic lake. Int J Syst Bacteriol 48:1405–1412
Moracci M, Ciaramella M, Rossi M (2001) β-Glycosidase from Sulfolobus solfataricus. Methods Enzymol 330:201–215
Nakamatu T, Yamanaka K (1969) Crystallization and properties of l-arabinose isomerase from Lactobacillus gayonii. Biochim Biophys Acta 178:156–165
Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Nelson WC, Ketchum KA, McDonald L, Utterback TR, Malek JA, Linher KD, Garrett MM, Stewart AM, Cotton MD, Pratt MS, Phillips CA, Richardson D, Heidelberg J, Sutton GG, Fleischmann RD, Eisen JA, White O, Salzberg SL, Smith HO, Venter JC, Fraser CM (1999) Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima. Nature 399:323–329
Patrick JW, Lee N (1968) Purification and properties of an l-arabinose isomerase from Escherichia coli. J Biol Chem 243:4312–4318
Pedersen S, Jørgensen OB (1995) Eur Patent 0502035
Petzelbauer I, Nidetzky B, Haltrich D, Kulbe KD (1999) Development of an ultra-high-temperature process for the enzymatic hydrolysis of lactose. I. The properties of two thermostable β-glycosidases. Biotechnol Bioeng 64:322–332
Petzelbauer I, Zeleny R, Reiter A, Kulbe KD, Nidetzky B (2000) Development of an ultra-high-temperature process for the enzymatic hydrolysis of lactose: II. Oligosaccharide formation by two thermostable β-glycosidases. Biotechnol Bioeng 69:140–149
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Siso MIG (1996) The biotechnological utilization of cheese whey: a review. Bioresour Technol 57:1–11
Sonne-Hansen J, Mathrani IM, Ahring BK (1993) Xylanolytic anaerobic thermophiles from Icelandic hot-springs. Appl Microbiol Biotechnol 38:537–541
Yamanaka K, Wood WA (1966) l-Arabinose isomerase. Methods Enzymol 9:596–602
Yoon S-H, Kim P, Oh D-K (2003) Properties of l-arabinose isomerase from Escherichia coli as biocatalyst for tagatose production. World J Microbiol Biotechnol 19:47–51
Acknowledgements
This work was supported by the Danish Ministry of Science, Technology and Development. We thank Bente Smith, Britt G. Olsen and Krisztina Faludi for skilful technical assistance, Robert P. Mortlock for generously providing a sample of A. aerogenes strain PRL-R3, Sven Pedersen for valuable advice, Qunxin She for generously providing a sample of purified DNA from Sulfolobus solfataricus and Rachel Kahn for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jørgensen, F., Hansen, O.C. & Stougaard, P. Enzymatic conversion of d-galactose to d-tagatose: heterologous expression and characterisation of a thermostable l-arabinose isomerase from Thermoanaerobacter mathranii . Appl Microbiol Biotechnol 64, 816–822 (2004). https://doi.org/10.1007/s00253-004-1578-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1578-6