Abstract
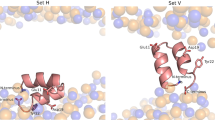
To better understand peptide-induced membrane fusion at a molecular level, we set out to determine the structure of the fusogenic peptide FP23 from the HIV-1 protein gp41 when bound to a lipid bilayer. An established solid-state 19F nuclear magnetic resonance (NMR) approach was used to collect local orientational constraints from a series of CF3-phenylglycine-labeled peptide analogues in macroscopically aligned membranes. Fusion assays showed that these 19F-labels did not significantly affect peptide function. The NMR spectra were characteristic of well-behaved samples, without any signs of heterogeneity or peptide aggregation at 1:300 in 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC). We can conclude from these NMR data that FP23 has a well-defined (time-averaged) conformation and undergoes lateral diffusion in the bilayer plane, presumably as a monomer or small oligomer. Attempts to evaluate its conformation in terms of various secondary structures, however, showed that FP23 does not form any type of regular helix or β-strand. Therefore, all-atom molecular dynamics (MD) simulations were carried out using the orientational NMR constraints as pseudo-forces to drive the peptide into a stable alignment and structure. The resulting picture suggests that FP23 can adopt multiple β-turns and insert obliquely into the membrane. Such irregular conformation explains why the structure of the fusion peptide could not be reliably determined by any biophysical method so far.







Similar content being viewed by others
References
Afonin S, Glaser RW, Berditchevskaia M, Wadhwani P, Guhrs KH, Mollmann U, Perner A, Ulrich AS (2003) 4-Fluorophenylglycine as a label for 19F-NMR structure analysis of membrane-associated peptides. Chembiochem 4:1151–1163
Afonin S, Dürr UHN, Glaser RW, Ulrich AS (2004) ‘Boomerang’-like insertion of a fusogenic peptide in a lipid membrane revealed by solid-state 19F NMR. Magn Reson Chem 42:195–203
Afonin S, Dürr UHN, Wadhwani P, Salgado JB, Ulrich AS (2008a) Solid state NMR structure analysis of the antimicrobial peptide gramicidin S in lipid membranes: concentration-dependent re-alignment and self-assembly as a β-barrel. Topics Curr Chem 273:139–154
Afonin S, Grage SL, Ieronimo M, Wadhwani P, Ulrich AS (2008b) Temperature-dependent transmembrane insertion of the amphiphilic peptide PGLa in lipid bilayers observed by solid state 19F-NMR spectroscopy. J Am Chem Soc 130:16512–16514
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) Tables of bond lengths determined by X-ray and neutron diffraction.1. Bond lengths in organic compounds. J Chem Soc Perkin Trans 2:S1–S19
Barz B, Wong TC, Kosztin I (2008) Membrane curvature and surface area per lipid affect the conformation and oligomeric state of HIV-1 fusion peptide: a combined FTIR and MD simulation study. Biochim Biophys Acta 1778:945–953
Bodner ML, Gabrys CM, Struppe JO, Weliky DP (2008) 13C–13C and 15 N–13C correlation spectroscopy of membrane-associated and uniformly labeled human immunodeficiency virus and influenza fusion peptides: amino acid-type assignments and evidence for multiple conformations. J Chem Phys 128:052319
Buchschacher GL Jr, Freed EO, Panganiban AT (1995) Effects of second-site mutations on dominant interference by a human immunodeficiency virus type 1 envelope glycoprotein mutant. J Virol 69:1344–1348
Castano S, Desbat B (2005) Structure and orientation study of fusion peptide FP23 of gp41 from HIV-1 alone or inserted into various lipid membrane models (mono-, bi- and multibi-layers) by FT-IR spectroscopies and Brewster angle microscopy. Biochim Biophys Acta 1715:81–95
Chang DK, Cheng SF, Chien WJ (1997a) The amino-terminal fusion domain peptide of human immunodeficiency virus type 1 gp41 inserts into the sodium dodecyl sulfate micelle primarily as a helix with a conserved glycine at the micelle-water interface. J Virol 71:6593–6602
Chang DK, Chien WJ, Cheng SF (1997b) The FLG motif in the N-terminal region of glucoprotein 41 of human immunodeficiency virus type 1 adopts a type-I beta turn in aqueous solution and serves as the initiation site for helix formation. Eur J Biochem 247:896–905
Chang DK, Cheng SF, Trivedi VD (1999) Biophysical characterization of the structure of the amino-terminal region of gp41 of HIV-1. Implications on viral fusion mechanism. J Biol Chem 274:5299–5309
Delahunty MD, Rhee I, Freed EO, Bonifacino JS (1996) Mutational analysis of the fusion peptide of the human immunodeficiency virus type 1: identification of critical glycine residues. Virology 218:94–102
Durell SR, Martin I, Ruysschaert JM, Shai Y, Blumenthal R (1997) What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion. Mol Membr Biol 14:97–112
Dürr UHN (2005) 19F-NMR studies on fluorine-labeled model compounds and biomolecules Biochemistry. Ph D thesis, University of Karlsruhe, Karlsruhe
Esteban-Martín S, Strandberg E, Fuertes G, Ulrich AS, Salgado J (2009) Influence of whole-body dynamics on 15N PISEMA NMR spectra of membrane peptides: a theoretical analysis. Biophys J 96:3233–3241
Esteban-Martín S, Strandberg E, Salgado J, Ulrich AS (2010) Solid state NMR analysis of peptides in membranes: influence of dynamics and labeling scheme. Biochim Biophys Acta 1798:252–257
Evans J, Morris GP (1990) Statistical mechanics of nonequilibrium liquids. Academic Press, London
Gabrys CM, Weliky DP (2007) Chemical shift assignment and structural plasticity of a HIV fusion peptide derivative in dodecylphosphocholine micelles. Biochim Biophys Acta 1768:3225–3234
Gerber D, Pritsker M, Gunther-Ausborn S, Johnson B, Blumenthal R, Shai Y (2004) Inhibition of HIV-1 envelope glycoprotein-mediated cell fusion by a DL-amino acid-containing fusion peptide: possible recognition of the fusion complex. J Biol Chem 279:48224–48230
Glaser RW, Grüne M, Wandelt C, Ulrich AS (1999) NMR and CD structural analysis of the fusogenic peptide sequence B18 from the fertilization protein bindin. Biochemistry 38:2560–2569
Glaser RW, Sachse C, Dürr UHN, Wadhwani P, Ulrich AS (2004) Orientation of the antimicrobial peptide PGLa in lipid membranes determined from 19F-NMR dipolar couplings of 4-CF3-phenylglycine labels. J Magn Reson 168:153–163
Glaser RW, Sachse C, Dürr UHN, Afonin S, Wadhwani P, Strandberg E, Ulrich AS (2005) Concentration-dependent realignment of the antimicrobial peptide PGLa in lipid membranes observed by solid-state 19F-NMR. Biophys J 88:3392–3397
Gordon LM, Mobley PW, Pilpa R, Sherman MA, Waring AJ (2002) Conformational mapping of the N-terminal peptide of HIV-1 gp41 in membrane environments using 13C-enhanced Fourier transform infrared spectroscopy. Biochim Biophys Acta 1559:96–120
Gordon LM, Mobley PW, Lee W, Eskandari S, Kaznessis YN, Sherman MA, Waring AJ (2004) Conformational mapping of the N-terminal peptide of HIV-1 gp41 in lipid detergent and aqueous environments using 13C-enhanced Fourier transform infrared spectroscopy. Protein Sci 13:1012–1030
Gordon LM, Nisthal A, Lee AB, Eskandari S, Ruchala P, Jung CL, Waring AJ, Mobley PW (2008) Structural and functional properties of peptides based on the N-terminus of HIV-1 gp41 and the C-terminus of the amyloid-beta protein. Biochim Biophys Acta 1778:2127–2137
Grage SL, Afonin S, Ulrich AS (2010) Dynamic transitions of membrane active peptides. Meth Mol Biol 618:183–207
Haque ME, Koppaka V, Axelsen PH, Lentz BR (2005) Properties and structures of the influenza and HIV fusion peptides on lipid membranes: implications for a role in fusion. Biophys J 89:3183–3194
Jaroniec CP, Kaufman JD, Stahl SJ, Viard M, Blumenthal R, Wingfield PT, Bax A (2005) Structure and dynamics of micelle-associated human immunodeficiency virus gp41 fusion domain. Biochemistry 44:16167–16180
Kanyalkar M, Srivastava S, Saran A, Coutinho E (2004) Conformational study of fragments of envelope proteins (gp120: 254–274 and gp41: 519–541) of HIV-1 by NMR and MD simulations. J Pept Sci 10:363–380
Kliger Y, Aharoni A, Rapaport D, Jones P, Blumenthal R, Shai Y (1997) Fusion peptides derived from the HIV type 1 glycoprotein 41 associate within phospholipid membranes and inhibit cell-cell fusion. Structure-function study. J Biol Chem 272:13496–13505
Li Y, Tamm LK (2007) Structure and plasticity of the human immunodeficiency virus gp41 fusion domain in lipid micelles and bilayers. Biophys J 93:876–885
Maddox MW, Longo ML (2002) Conformational partitioning of the fusion peptide of HIV-1 gp41 and its structural analogs in bilayer membranes. Biophys J 83:3088–3096
Maisch D, Wadhwani P, Afonin S, Koksch B, Ulrich AS (2009) Chemical labeling strategy with (R)- and (S)-triofluoromethylalanin for solid state 19F-NMR analysis of peptaibols in membranes. J Am Chem Soc 131:15596–15597
Marsan MP, Muller I, Ramos C, Rodriguez F, Dufourc EJ, Czaplicki J, Milon A (1999) Cholesterol orientation and dynamics in dimyristoylphosphatidylcholine bilayers: a solid state deuterium NMR analysis. Biophys J 76:351–359
Mobley PW, Waring AJ, Sherman MA, Gordon LM (1999) Membrane interactions of the synthetic N-terminal peptide of HIV-1 gp41 and its structural analogs. Biochim Biophys Acta 1418:1–18
Mykhailiuk PK, Afonin S, Chernega AN, Rusanov EB, Platonov MO, Dubinina GG, Berditsch M, UIrich AS, Komarov IV (2006) Conformationally rigid trifluoromethyl-substituted α-amino acid designed for peptide structure analysis by solid-state 19F NMR spectroscopy. Angew Chem Int Ed Engl 45:5659–5661
Mykhailiuk PK, Afonin S, Palamarchuk GV, Shishkin OV, Ulrich AS, Komarov IV (2008) Synthesis of trifluoromethyl-substituted proline analogues as 19F NMR labels for peptides in the polyproline II conformation. Angew Chem Int Ed Engl 47:5765–5767
Peisajovich SG, Epand RF, Pritsker M, Shai Y, Epand RM (2000) The polar region consecutive to the HIV fusion peptide participates in membrane fusion. Biochemistry 39:1826–1833
Pereira FB, Goni FM, Muga A, Nieva JL (1997) Permeabilization and fusion of uncharged lipid vesicles induced by the HIV-1 fusion peptide adopting an extended conformation: dose and sequence effects. Biophys J 73:1977–1986
Peuvot J, Schanck A, Lins L, Brasseur R (1999) Are the fusion processes involved in birth, life and death of the cell depending on tilted insertion of peptides into membranes? J Theor Biol 198:173–181
Pritsker M, Jones P, Blumenthal R, Shai Y (1998) A synthetic all D-amino acid peptide corresponding to the N-terminal sequence of HIV-1 gp41 recognizes the wild-type fusion peptide in the membrane and inhibits HIV-1 envelope glycoprotein-mediated cell fusion. Proc Natl Acad Sci U S A 95:7287–7292
Pritsker M, Rucker J, Hoffman TL, Doms RW, Shai Y (1999) Effect of nonpolar substitutions of the conserved Phe11 in the fusion peptide of HIV-1 gp41 on its function, structure, and organization in membranes. Biochemistry 38:11359–11371
Qiang W, Weliky DP (2009) HIV fusion peptide and its cross-linked oligomers: efficient syntheses, significance of the trimer in fusion activity, correlation of beta strand conformation with membrane cholesterol, and proximity to lipid headgroups. Biochemistry 48:289–301
Qiang W, Yang J, Weliky DP (2007) Solid-state nuclear magnetic resonance measurements of HIV fusion peptide to lipid distances reveal the intimate contact of beta strand peptide with membranes and the proximity of the Ala-14-Gly-16 region with lipid headgroups. Biochemistry 46:4997–5008
Qiang W, Bodner ML, Weliky DP (2008) Solid-state NMR spectroscopy of human immunodeficiency virus fusion peptides associated with host-cell-like membranes: 2D correlation spectra and distance measurements support a fully extended conformation and models for specific antiparallel strand registries. J Am Chem Soc 130:5459–5471
Qiang W, Sun Y, Weliky DP (2009) A strong correlation between fusogenicity and membrane insertion depth of the HIV fusion peptide. Proc Natl Acad Sci U S A 106:15314–15319
Rafalski M, Lear JD, DeGrado WF (1990) Phospholipid interactions of synthetic peptides representing the N-terminus of HIV gp41. Biochemistry 29:7917–7922
Reichert J, Grasnick D, Afonin S, Buerck J, Wadhwani P, Ulrich AS (2007) A critical evaluation of the conformational requirements of fusogenic peptides in membranes. Eur Biophys J 36:405–413
Sackett K, Shai Y (2002) The HIV-1 gp41 N-terminal heptad repeat plays an essential role in membrane fusion. Biochemistry 41:4678–4685
Sackett K, Shai Y (2003) How structure correlates to function for membrane associated HIV-1 gp41 constructs corresponding to the N-terminal half of the ectodomain. J Mol Biol 333:47–58
Sackett K, Nethercott M, Shai Y, Weliky D (2009) Hairpin folding of HIV gp41 abrogates lipid mixing function at physiologic pH and inhibits lipid mixing by exposed gp41 constructs. Biochemistry 48:2714–2722
Sackett K, Nethercott MJ, Epand RF, Epand RM, Kindra DR, Shai Y, Weliky DP (2010) Comparative analysis of membrane-associated fusion peptide secondary structure and lipid mixing function of HIV gp41 constructs that model the early pre-hairpin intermediate and final hairpin conformations. J Mol Biol 397:301–315
Salgado J, Grage SL, Kondejewski LH, Hodges RS, McElhaney RN, Ulrich AS (2001) Membrane-bound structure and alignment of the antimicrobial β-sheet peptide gramicidin S derived from angular and distance constraints by solid state 19F-NMR. J Biomol NMR 21:191–208
Sternberg U, Koch F-T, Losso P (2006) COSMOS program. COSMOS Software, Jena
Sternberg U, Witter R, Ulrich AS (2007) All-atom molecular dynamics simulations using orientational constraints from anisotropic NMR samples. J Biomol NMR 38:23–39
Strandberg E, Ulrich AS (2004) NMR methods for studying membrane-active antimicrobial peptides. Concepts Magn Reson A 23A:89–120
Strandberg E, Özdirekcan S, Rijkers DTS, Van der Wel PCA, Koeppe RE, II, Liskamp RMJ, Killian JA (2004) Tilt angles of transmembrane model peptides in oriented and non-oriented lipid bilayers as determined by 2H solid state NMR. Biophys J 86:3709–3721
Strandberg E, Wadhwani P, Tremouilhac P, Dürr UHN, Ulrich AS (2006) Solid-state NMR analysis of the PGLa peptide orientation in DMPC bilayers: structural fidelity of 2H-labels versus high sensitivity of 19F-NMR. Biophys J 90:1676–1686
Strandberg E, Kanithasen N, Tiltak D, Bürck J, Wadhwani P, Zwernemann O, Ulrich AS (2008) Solid-state NMR analysis comparing the designer-made antibiotic MSI-103 with its parent peptide PGLa in lipid bilayers. Biochemistry 47:2601–2616
Strandberg E, Esteban-Martín S, Salgado J, Ulrich AS (2009) Orientation and dynamics of peptides in membranes calculated from 2H-NMR data. Biophys J 96:3223–3232
Tremouilhac P, Strandberg E, Wadhwani P, Ulrich AS (2006a) Conditions affecting the re-alignment of the antimicrobial peptide PGLa in membranes as monitored by solid state 2H-NMR. Biochim Biophys Acta 1758:1330–1342
Tremouilhac P, Strandberg E, Wadhwani P, Ulrich AS (2006b) Synergistic transmembrane alignment of the antimicrobial heterodimer PGLa/magainin. J Biol Chem 281:32089–32094
Ulrich AS (2005) Solid state 19F-NMR methods for studying biomembranes. Progr Nucl Magn Reson Spectrosc 46:1–21
Ulrich AS (2007) Solid state 19F-NMR analysis of oriented biomembranes. In: Webb GA (ed) Modern magnetic resonance, vol 1. Springer, Dordrecht, pp 261–267
Ulrich AS, Tichelaar W, Förster G, Zschörnig O, Weinkauf S, Meyer HW (1999) Ultrastructural characterization of peptide-induced membrane fusion and peptide self-assembly in the bilayer. Biophys J 77:829–841
Ulrich AS, Wadhwani P, Dürr UHN, Afonin S, Glaser RW, Strandberg E, Tremouilhac P, Sachse C, Berditchevskaia M, Grage SL (2006) Solid-state 19F-nuclear magnetic resonance analysis of membrane-active peptides. In: Ramamoorthy A (ed) NMR spectroscopy of biological solids. CRC Press, Boca Raton, pp 215–236
Wadhwani P, Bürck J, Strandberg E, Mink C, Afonin S, Ulrich AS (2008) Using a sterically restrictive amino acid as a 19F-NMR label to monitor and control peptide aggregation in membranes. J Am Chem Soc 130:16515–16517
Wadhwani P, Reichert J, Bürck J, Ulrich AS (2010) Antimicrobial and cell penetrating peptides can trigger membrane fusion by folding and aggregation (submitted)
Wasniewski CM, Parkanzky PD, Bodner ML, Weliky DP (2004) Solid-state nuclear magnetic resonance studies of HIV and influenza fusion peptide orientations in membrane bilayers using stacked glass plate samples. Chem Phys Lipids 132:89–100
White JM, Delos SE, Brecher M, Schornberg K (2008) Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit Rev Biochem Mol Biol 43:189–219
Wong TC (2003) Membrane structure of the human immunodeficiency virus gp41 fusion peptide by molecular dynamics simulation. II. The glycine mutants. Biochim Biophys Acta 1609:45–54
Yang J, Weliky DP (2003) Solid-state nuclear magnetic resonance evidence for parallel and antiparallel strand arrangements in the membrane-associated HIV-1 fusion peptide. Biochemistry 42:11879–11890
Yang J, Gabrys CM, Weliky DP (2001) Solid-state nuclear magnetic resonance evidence for an extended β strand conformation of the membrane-bound HIV-1 fusion peptide. Biochemistry 40:8126–8137
Yang R, Yang J, Weliky DP (2003) Synthesis, enhanced fusogenicity, and solid state NMR measurements of cross-linked HIV-1 fusion peptides. Biochemistry 42:3527–3535
Yang R, Prorok M, Castellino FJ, Weliky DP (2004) A trimeric HIV-1 fusion peptide construct which does not self-associate in aqueous solution and which has 15-fold higher membrane fusion rate. J Am Chem Soc 126:14722–14723
Zheng Z, Yang R, Bodner ML, Weliky DP (2006) Conformational flexibility and strand arrangements of the membrane-associated HIV fusion peptide trimer probed by solid-state NMR spectroscopy. Biochemistry 45:12960–12975
Acknowledgments
We thank Johannes Reichert and Jochen Bürck for their help with the lipid mixing assays, dynamic light scattering measurements, and CD spectroscopy, Sergii Afonin and Olaf Zwernemann for their advice on peptide synthesis and purification, Igor Jakovkin for performing the statistical analysis on the peptide backbone, and Carl Philipp Ulrich for his constructive comments. The project was partially funded by the DFG-Center for Functional Nanostructures (E1.2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Membrane-active peptides: 455th WE-Heraeus-Seminar and AMP 2010 Workshop.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grasnick, D., Sternberg, U., Strandberg, E. et al. Irregular structure of the HIV fusion peptide in membranes demonstrated by solid-state NMR and MD simulations. Eur Biophys J 40, 529–543 (2011). https://doi.org/10.1007/s00249-011-0676-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0676-5