Abstract
Ammonia oxidising archaea (AOA) are ecologically important nitrifiers in acidic agricultural soils. Two AOA phylogenetic clades, belonging to order-level lineages of Nitrososphaerales (clade C11; also classified as NS-Gamma-2.3.2) and family-level lineage of Candidatus Nitrosotaleaceae (clade C14; NT-Alpha-1.1.1), usually dominate AOA population in low pH soils. This study aimed to investigate the effect of different fertilisation histories on community composition and activity of acidophilic AOA in soils. High-throughput sequencing of ammonia monooxygenase gene (amoA) was performed on six low pH agricultural plots originating from the same soil but amended with different types of fertilisers for over 20 years and nitrification rates in those soils were measured. In these fertilised acidic soils, nitrification was likely dominated by Nitrososphaerales AOA and ammonia-oxidising bacteria, while Ca. Nitrosotaleaceae AOA activity was non-significant. Within Nitrososphaerales AOA, community composition differed based on the fertilisation history, with Nitrososphaerales C11 only representing a low proportion of the community. This study revealed that long-term soil fertilisation selects for different acidophilic nitrifier communities, potentially through soil pH change or through direct effect of nitrogen, potassium and phosphorus. Comparative community composition among the differently fertilised soils also highlighted the existence of AOA phylotypes with different levels of stability to environmental changes, contributing to the understanding of high AOA diversity maintenance in terrestrial ecosystems.
Similar content being viewed by others
Introduction
Acidic soils consist of almost 30% of the world’s land area and half of arable fields [1]. In the past decade, soil acidity has been intensified due to increasing amount of ammonium-based fertilisers applied in agricultural lands in China and globally [2]. Although reduced soil pH impairs many belowground biochemical processes [3], nitrification appears not to be affected as similar rates occurred in acidic and neutral soils [4]. Nevertheless, there is evidence for pH niche specialisation of ammonia oxidisers [5, 6]. The initial and rate limiting step of nitrification, the conversion of ammonia to nitrite, is performed by ammonia oxidising archaea (AOA), canonical bacteria (AOB) and more recently discovered complete ammonia bacterial oxidisers (commamox). While knowledge on comammox distribution and activity in soils is still scarce, AOA rather than AOB activity was shown to control ammonia oxidation in most of the low pH soils [7,8,9,10]. In contrast, both AOA and AOB appeared active in neutral to alkaline soils [11, 12], despite some recent evidence of AOB activity in acidic soils [13, 14]. It was further demonstrated that soil pH might determine the niche specialisation of diverse phylogenetic clades, both for AOA [5] and AOB [15]. AOA are phylogenetically placed into clades from three orders, including Nitrososphaerales, Nitrosopumilales and Candidatus Nitrosocaldales [16]. Several AOA taxonomic rankings based on the key functional gene, ammonia monooxygenase amoA, have been proposed in the literature and the one focusing on terrestrial AOA [5] was chosen in the present study (with correspondence with a global AOA classification [17] being presented in Fig. 1). Among the phylogenetically well characterised 19 AOA clades (cluster 1–19, C1-C19), two clades are particularly abundant in acidic and acido-neutral soils (C11 within the order-level lineage of Nitrososphaerales and C14 within the family-level lineage of Ca. Nitrosotaleaceae), while others, less abundant, also appear well-adapted to such environments (e.g. Nitrososphaerales C6 or some members of Nitrososphaerales C13) [18]. Many ecological studies have confirmed high abundance or activity of Nitrososphaerales and/or Ca. Nitrosotaleaceae AOA in acidic soils [9, 19, 20], but the environmental factors regulating their distribution are still not elucidated.
Heatmap displaying the relative abundance of different phylogenetic clades of ammonia oxidising archaea within the class Nitrososphaeria, estimated by pyrosequencing of archaeal amoA gene fragments. The phylogeny of different clades was constructed using known representative amoA gene sequences from each clade. Names of the phylogenetic clades include initial terrestrial denomination (C1–C19) [18] and more recent denomination of these clades is indicated in brackets [17] to unify the two phylogenetic approaches. The colour of a phylogenetic clade name indicates the pH specialisation of the clade from previous meta-analysis at a global scale [18]. Soils were ranked from low to high pH conditions (from left to right). Different letters (a–c) next to the relative abundance values for clades C1, C2 and C6 indicate significant difference in relative abundance between soils with different fertilisation history (p ≤ 0.05)
Ammonium-based N fertilisers might stimulate different groups of ammonia oxidisers, due to potential distinct affinities to and/or competitivity for substrate [21,22,23]. Therefore, this study aimed to test the hypothesis that different fertilisation sources also influence the AOA nitrifier community distribution in low pH agricultural soils. Indeed, it was previously observed that long-term N fertilisation affected AOA rather than AOB community compositions in low pH fields [24]. Specifically, while Ca. Nitrosotaleaceae AOA were observed with no amendment of nitrogen (N), phosphorus (P) or potassium (K) elements, soils amended with all those three elements (NPK) contained exclusively Nitrososphaerales-affiliated AOA [24]. This suggests that nutrient element limitation might be an important niche differential factor for acid-tolerant or acidophilic AOA groups, with the implication that Nitrososphaerales AOA are more competitive than Ca. Nitrosotaleaceae AOA under multiple nutrient enriched condition (i.e. fertilised with NPK or organic manure compost). To test this hypothesis, we characterised the AOA community in several low pH agricultural soils being subject to different fertilisation managements for > 20 years using high-throughput sequencing of the archaeal ammonia monooxygenase (amoA) gene. We predict that (i) AOA community is dominated by Ca. Nitrosotaleaceae and Nitrososphaerales AOA and more specifically by acidophilic clades of C14 and C11, and (ii) the relative abundance of Nitrososphaerales AOA will increase with multiple nutrient supplies (with NPK or manure amendment). In addition, this study aimed to identify the environmental variables potentially affecting the community composition and provide evidence for the distinct relative contribution of different ammonia oxidiser groups (Nitrososphaerales, Ca. Nitrosotaleaceae AOA and AOB) to nitrification in acidic agricultural soils under different fertilisation regimes.
Materials and Methods
Site Description and Soil Sampling
The long-term fertilisation experiment fields are located at the Ecology Experimental Station of Red Soil, Chinese Academy of Sciences (28° 15′ N, 116° 55′ E) in the city of Yingtan, province Jiangxi of China. This region has a typical subtropical monsoon climate with a mean annual precipitation of 1785 mm and a mean annual temperature of 18 °C. Soil derives from quaternary red clay and is classified as Hapludults. The fertilisation experiment was initially established in 1989 with a peanut-rutabaga rotation system, and since 1995 was maintained as cropping of peanut with fallow in winter. Soil samples were collected from different fields receiving different fertilisation regimes with descriptions as follows: (1) PK, plots without N fertilisation but amended with P and K; (2) NP, plots receiving chemical N fertiliser and amended with P; (3) NK, plots receiving chemical N fertiliser and amended with K; (4) NPK, plots receiving chemical N fertilisers and amended with P and K; (5) 1/2NPK, plots receiving same types of fertilisers as in NPK plots but with half amount; and (6) 1/2NPK + OM, plots receiving the same fertilisers as in 1/2NPK plots plus additional organic manure. The annual application rates of N, P and K were 120 kg urea-N/hm2, 40 kg P2O5-P/hm2 and 118 kg K2O-K/hm2, respectively, and the 1/2NPK + OM received additional 15,000 kg/hm2 swine manure in addition to 1/2NPK fertilisation. Each treatment was applied to three field plots and soil was collected in each plot as one biological replicate. For each biological replicate, soil samples at 0–20 cm depth were collected during fallow period within each plot from five random cores (with distance of 2–3 m between cores) in May 2012, sieved through a 2.0-mm sieve and homogenized to make a composite sample. The subsamples were kept at 4 °C until analysis for soil physiochemical analysis, and at − 20 °C for genomic DNA extraction.
Soil Physiochemical Analysis
Soil pH was determined using a soil-to-water ratio of 1:5 with a Mettler Toledo 320-S pH meter (Mettler–Toledo Instruments Co. Ltd., Shanghai, China). Soil organic matter and total N were determined by dichromate oxidation method and Kjeldahl digestion method, respectively. Soil NH4+ and NO3− concentrations were determined using a Skalar SAN Plus Segmented Flow Analyser (Skalar Inc., Breda, Netherlands) after extraction with 2 M KCl solution. Soil available P was extracted by sodium bicarbonate and analysed using the molybdenum blue method. Soil available K was extracted by ammonium acetate and estimated by flame photometry.
Nucleic Acid Extraction and Pyrosequencing
Soil DNA was extracted using a FastDNA spin kit for soil (MP Biomedicals, Cleveland, OH, USA), according to the manufacturer’s instruction. The DNA quantity and purity were determined by a Nanodrop ND-1000 UV–Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and diluted to 2–10 ng μl−1 for following molecular tests.
High-throughput sequencing of the amoA gene was performed using the primers CrenamoA 23f/CrenamoA 616r [25] with the forward primer containing unique barcode sequences for demultiplexing samples. PCR reaction was performed in a 50 µl mixture containing 25 µl of Platinum PCR SuperMix (Invitrogen, Shanghai, China), 1 µM of each primer and 2 µl of DNA template. The thermal condition was as follows: 94 °C for 3 min, 35 cycles of 94 °C for 45 s, 55 °C for 30 s and 72 °C for 90 s, followed by extension at 72 °C for 5 min. Negative control using sterile water instead of soil DNA was always included. PCR products were gel-purified and quantified using Picogreen dsDNA Quantitation Kits (Invitrogen). Adapter sequence was added to the forward end of the PCR amplicon fragments before performing the sequencing on a Roche FLX 454 pyrosequencing machine (Roche Diagnostics Corporation, Branford, CT).
The bioinformatic analyses of amplicon sequencing of marker genes included following key steps. Forward sequence reads were truncated at 350 bp and reads were demultiplexed and quality filtered using Mothur [26]. Reads with average quality score < 25, mismatched primers, ambiguous bases and frameshift errors were removed as described in a previously study [15]. The resultant sequences were assigned to a database of phylogenetic amoA gene clades (C1–C19) [5] using a BLASTn approach (www.ncbi.nlm.nih.gov).
Quantification of Ammonia Oxidisers
Real-time quantitative PCR (qPCR) was performed to determine archaeal and bacterial amoA gene abundances on a CFX96 Optical Real-Time Detection System (Bio-Rad, Laboratories Inc., CA, USA). Primer pair Arch-amoAF/Arch-amoAR [27] was used and qPCR reaction was performed in a 25 µl volume containing 12.5 µl SYBR Premix Ex Taq (TaKaRa Biotechnology, Shiga, Japan), 1 μM of each primer and 2 μl of tenfold diluted DNA template (1–10 ng). Amplification conditions were as follows: 95 °C for 1 min, 40 cycles of 10 s at 95 °C, 30 s at 55 °C, 1 min at 72 °C, followed by plate reads at 83 °C. Bacterial amoA gene was quantified using the primers amoA1F/amoA2R [28], with the same PCR conditions used for archaeal amoA gene quantification. The standards were generated and used as previously described [8]. qPCR was performed in biological triplicate each containing 3 technical replicates. Amplification efficiency was 98–103% with R2 values > 0.99. The specificity of real-time PCR amplification was confirmed by melting curve analysis and agarose gel electrophoresis at the end of each qPCR run.
Nitrification Activity by 15 N-Isotope Tracing Microcosms
15 N-isotope tracing was exploited to estimate nitrification activity under near-in situ incubation conditions. The incubation was established in triplicate in 250 ml Erlenmeyer flask containing 10 g of the sieved fresh soil from each plot. The 15 N labelled urea (15 N atom > 98%) was applied to the soil at a final concentration of 5 μg N g−1 dry soil, which approximated to the NH4+-N concentration in soils under in situ condition. The soil was incubated at 25 °C in darkness for 7 days in the absence or presence of 0.1% (v/v) acetylene, an inhibition gas for ammonia oxidation. The production of soil 15 N-labelled nitrate and nitrite (NOx−) after 7-day incubation was measured and calculated as previously described [8, 29].
Statistical Analysis
The putative relative ammonia oxidiser contribution to 15NOx− production was estimated according to the ammonia oxidiser abundance and their recorded cell-specific activity. Firstly, the relative abundance of Nitrososphaerales and Ca. Nitrosotaleaceae AOA was calculated based on high-throughput sequencing of amoA genes. The cell abundance of Nitrososphaerales and Ca. Nitrosotaleaceae AOA was then calculated by multiplying the relative abundance by the total AOA abundance estimated by qPCR of amoA genes. The cell abundance of AOB was calculated as the bacterial amoA gene abundance divided by 2.5, considering that one AOB cell contains on average 2.5 copies of amoA genes [30]. The putative proportions of Nitrososphaerales AOA, Ca. Nitrosotaleaceae AOA and AOB in the 15NOx− production were then estimated according to previous record of specific cell activity of 2.6 fmol NH3 cell−1 h−1 for Nitrososphaera viennensis [31], 0.072 fmol NH3 cell−1 h−1 for Ca. Nitrosotalea devanaterra [32], and 23 fmol NH3 cell−1 h−1 for Nitrosospira multiformis [33], respectively. The specific cell activity records used here are from pure cultures under optimal cultivation conditions, and the relative contribution to the nitrification was estimated assuming they all reached these records in the soils as previously described [13].
Statistical analysis was performed on Statistics 23.0 (SPSS, IL, USA). One-way ANOVA was employed to determine the effect of different fertilisation treatments on soil 15NOx− production rate and relative abundance of each AOA clade (C1-C19), followed by a Tukey post hoc test to determine significant mean differences. Alpha diversity indices, including number of OTUs and Shannon’s index, were calculated after random sampling of 400 reads per sample following OTU clustering at 100% sequence identity. Non-metric multidimensional scaling (NMDS) based on a Bray–Curtis dissimilarity matrix was performed on AOA composition using the vegan package under R environment, and analysis of similarity (ANOSIM) was used to assess the variations between soil sites [34]. Function ‘envfit’ was used to test the significance of chemical properties (as vectors) and fertilisation treatments (as factors) for the NMDS ordinations, with 999 permutations using vegan [34]. Differences at p < 0.05 were considered statistically significant.
Results
Changes in Soil Properties
Soil pH was affected by different fertilisation regimes. The pH was lower in most soils receiving chemical N fertilisers (pH 4.2–5.2; NK, NP, NPK and 1/2NPK) compared to the soil with no N fertilisation history (pH 5.7; PK), to the exception of the soil amended with additional supply of organic manure which had the highest pH (pH 6.3; 1/2NPK + OM) (Table 1). The soil with manure fertilisation also had the highest contents of soil organic matter (SOM), total N, inorganic N (both NH4+ and NOx−) and available P, whereas soil treatment NK had the lowest total N, inorganic N and available P concentration (Table 1). Additionally, available K contents were the highest and the lowest in PK and NP treatments, respectively (Table 1).
Compositional Change of Ammonia Oxidising Archaea
Pyrosequencing of archaeal amoA gene fragments generated 81,958 raw sequences from all sites, with an average of 5061 raw reads (range between 2092 and 10,011 sequences) per sample. Two replicates from PK and 1/2NPK soils resulted in only 942 and 43 sequences, respectively, and were therefore removed from further analysis. Quality filtering of the sequences resulted in a total of 16,590 high-quality sequences from all sites, accounting for 20.2% of all original sequences. All AOA amoA sequences affiliated to the Nitrososphaerales and Ca. Nitrosotaleaceae lineages (Fig. 1 and Fig. S1), with Nitrososphaerales accounting for 88.4–100% of amoA gene sequences in different soils. Nitrososphaerales C6, C1, C2 and C3 clades were the most abundant phylogenetic clades accounting for up to 83.8%, 53.0%, 32.8% and 15.5% of the AOA population, respectively (Fig. 1). In contrast, Ca. Nitrosotaleaceae C14 accounted for up to 11.6% of the AOA population (Fig. 1). Within Nitrososphaerales, the relative abundance of the C1, C2 and C6 clades depended on the soil fertilisation histories, with C1 and C2 having the highest relative abundance in soils with no N fertilisation (PK) or extra organic manure fertilisation (1/2NPK + OM), which were the soils with the highest pH (Table 1), while C6 relative abundance was highest in the other soils (Fig. 1). Due to the shifts in the relative abundance of different AOA clades, AOA compositions were dissimilar in soils with different fertilisation histories, based on NMDS analysis (Fig. 2). Different fertilisation treatments had a significant effect on the AOA community compositions between higher (PK and 1/2NPK + OM, pH 5.7–6.3) and lower (NK, NP, NPK and 1/2NPK, pH 4.2–5.2) pH soil groups (p = 0.002) following ANOSIM analysis based on the Bray–Curtis dissimilarity. Multivariate analyses showed that change in soil pH most strongly correlated with variation in AOA compositions among all tested environmental variables (Fig. 2 and Table S1). The alpha diversity indices of AOA, including richness (number of OTUs) and evenness (Shannon’s index), were higher in PK and 1/2NPK + OM soils than the other soils, although the difference was not significant for the richness index (Fig. S2).
Nonmetric multidimensional scaling plot of archaeal ammonia oxidiser community compositions in different soils based on normalized abundance of different AOA clades. The soils were ranked from low to high pH conditions (from top to bottom) in the figure legend. The association of measured environmental variables was shown as the grey line segments in the plot using ‘envfit’ function, with the length of the lines proportional to the correlations between environmental variables and the ordination values and the direction pointing to increasing values of the environmental variables
Ammonia Oxidiser Abundance
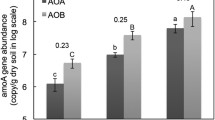
Both AOA and AOB were detected in all soils by quantification of amoA genes. AOA gene abundance ranged between 4.9 × 107 and 1.3 × 108 per g of soil, with the highest abundance being estimated in the soils with higher pH (Fig. 3a). After multiplying the total AOA gene abundance by the relative abundance of Nitrososphaerales and Ca. Nitrosotaleaceae (based on the pyrosequencing analysis), the abundance of these two clades was estimated to be 4.4 × 107–1.3 × 108 and 0–6.6 × 106 per g of soil, respectively (Fig. 3a). AOB gene abundance was significantly lower than AOA abundance in all soils, ranging from 1.4 × 106 to 3.7 × 107 per g of soil (Fig. 3a).
Abundance (a) and putative contribution (b) of Nitrososphaerales AOA, Ca. Nitrosotaleaceae AOA and betaproteobacterial AOB to nitrification. a Total AOA and AOB abundances were estimated by qPCR of amoA genes, and Nitrososphaerales AOA and Ca. Nitrosotaleaceae AOA abundances were calculated by multiplying total AOA abundance by the relative abundance of each AOA lineage. b Nitrification activity was estimated as the production rate of 15NOx−-N converted from 15 N-urea following 7-day microcosm incubation and the putative contributions were estimated using specific cell activity of 2.6, 0.072 and 23 fmol NH3 cell−1 h−1 for Nitrososphaerales AOA, Ca. Nitrosotaleaceae AOA and AOB, respectively. Soils were ranked from low to high pH conditions (from left to right). Error bars represent standard errors of means from triplicate microcosms and different letters above the bars indicate statistically significant differences (p ≤ 0.05) within each plot
Nitrification Activity and Putative AO Contributions to Soil Nitrification
Soil nitrification activity was assessed by temporal increase in 15 N-NOx− content converted from 15 N-NH4+ amended in the microcosms after 7-day incubation. The highest nitrification activity was observed in the soil receiving organic manure (1/2NPK + OM treatment), while the lowest activity was detected in NK and NP soils (Fig. 3b). Acetylene fully inhibited nitrate production in all soil microcosms (Fig. S3). Putative ammonia oxidiser contributions to the nitrification rate were then estimated according to the ammonia oxidiser abundance and the recorded AOA and AOB cell-specific activity. Nitrososphaerales AOA and AOB were putatively the major contributors to nitrification in these soils, accounting for 38.9–89.5% and 10.1–60.9% of the nitrification rates, respectively, while Ca. Nitrosotaleaceae AOA contribution was negligible (0–0.4% of the rates) (Fig. 3b).
Soil 15NOx− production rate was positively correlated with archaeal amoA gene abundance (polynomial best-fitting model: y = 3e−14x2 – 4e−06x + 173, p = 0.042) (Fig. 4b) but not with bacterial amoA gene abundance (p = 0.343) (Fig. 4b). Soil 15NOx− production rate also positively correlated with soil pH (polynomial best-fitting model: y = 34x2 – 304x + 723, p = 0.014) (Fig. 4c). Additionally, soil pH positively correlated with AOA abundance but not AOB abundance (Fig. S4).
Correlations of 15 N-NO3− production rate with AOA abundance (a), AOB abundance (b) and soil pH (c). AOA and AOB abundances were estimated by qPCR of archaeal and bacterial amoA genes, respectively. Error bars represent standard errors of means from triplicate microcosms and regression coefficients of the best fitting model and associated p values are indicated. Small standard error values were hidden by the mean value symbols
Discussion
The Ecology of Nitrososphaerales and Ca. Nitrosotaleaceae AOA in Acidic Agricultural Soils
This study predicted that Nitrososphaerales and Ca. Nitrosotaleaceae clades dominate AOA population in the low pH agricultural soils, especially the clades of C11 and C14, which are the most abundant AOA clades in acidic soils based on a global database study [5, 35]. Unexpectedly, neither C11 nor C14 was retrieved as the main AOA nitrifiers in our soils, indicating that strong physiological adaptation to low soil pH was not restricted to these previously recognized AOA clades. Nonetheless, the relative abundance of C14 seemed to increase in the soils with a decreased pH (especially in NK soil, pH 4.2, Fig. 1), despite no statistical difference observed between different soils due to large replication variations in this study. This suggested great adaptability of C14 to strongly acidic environment (pH < 4.5), being consistent with previous studies showing strong AO activity of this clade in such soils (see list of SIP experiments in Table 2). Additionally, previous studies on acidic red soils from the same region (Yingtan, see Table 2) estimated that Nitrososphaerales C11 were the most abundant AOA in some forest soils while they were not detected in reclaimed agricultural soils, suggesting that this AOA clade might not be favoured under agricultural management. This might explain the generally low proportion of Nitrososphaerales C11 in all agricultural soils used in this study.
Instead of Nitrososphaerales C11 and Ca. Nitrosotaleaceae C14, AOA population was dominated by Nitrososphaerales C3, C6 or C1/C2 clades in our soils. However, these AOA clades had different levels of adaptability to different fertilisation histories, supposedly leading to distinct AOA compositions in these soils (Fig. 2). Nitrososphaerales clade C3 were detected at similar proportions in different soils, irrespective of different fertilisation history (Fig. 1 and Fig. S1), suggesting nitrifiers within this clade adapt to a wide range of environmental conditions and form the common and widespread archaeal nitrifiers in these soils. This consistently detected AOA clade might be considered ‘habitat generalist’ with similar distribution pattern in these soils [36], which can play a key role in maintenance of taxonomic diversity [37] and may have strong potential for dormancy [38]. In comparison, C6 and C1/2 showed higher sensitivity to environmental changes, as C6 were most abundant in lower pH soils (NK, NP, NPK and 1/2NPK, pH 4.2–5.2) and C1/2 dominated in more neutral pH soils (PK and 1/2NPK + OM, pH 5.7–6.3) (Fig. 1 and Fig. S1). The compensation of C1/C2 abundance by C6 in lower pH soils suggests redundant function of distinct AOA phylotypes in soils, which might facilitate fast recovery of nitrification rate following environmental changes [39]. Similar trend was observed from a list of literatures (Table 2), as C1/C2 clades only dominated AOA population in soils with pH ≥ 5.7 and the C6 were often predominant in lower pH soils (4.0–5.3). In addition, Nitrososphaerales clade C10 was abundantly detected in many lower pH soils from previous studies, which was not observed in our soils. Interestingly, based on DNA-based stable isotope probing studies, the most actively growing Nitrososphaerales clades in these low pH agricultural soils were restricted to clades of C1/C2, C3 and C11 (Table 2) [9, 19, 40]. This indicated the lifestyles and metabolic traits of different AOA clades might be distinct. While millions years of evolution separate these AOA clades and associated metabolic traits [5, 35], AOA adaptation to such acidic conditions was likely facilitated by acquisition of V-type ATPases via horizontal transfer [41] or other traits linked to pH homeostasis [42].
Influence of Different Fertilisation Histories on AOA Composition and Abundance
We also hypothesized that proportion of Nitrososphaerales AOA would be greater in the low pH agricultural soils receiving higher nutrient supplies of NPK or manure amendment. However, this study did not observe significant difference of Nitrososphaerales proportions between soils receiving all major nutrient elements (NPK, 1/2NPK and 1/2NPK + OM) and those with one nutrient element unamended (NP, NK, PK). Different nutrient amendments appeared to have no direct impact on the AOA community compositions, but the consequent change in soil pH condition might be one of the key drivers, according to correlation test on several environmental variables (Fig. 2). Indeed, pH could roughly classify the soils into two main groups, one with more acidic pH range (NK, NP, NPK and 1/2NPK, pH ≤ 5.2) having closer AOA community structure compared to the higher pH soil group (PK and 1/2NPK + OM, pH 5.7–6.3) (Fig. 2). These two groups of soils were also dominated by distinct AOA clades as discussed above and had different AOA diversity indices (Fig. S2). Some nutrient factors, e.g. soil NH4+ concentration, also showed consistency with AOA composition based on multivariate analyses (Fig. 2). However, their role in shaping AOA community assembly is uncertain, as most of these factors are fluctuating with agricultural management.
Although the multiple nutrient supplies might not determine the composition of AOA in our soils, the amendment or lack of certain nutrient element might affect the ammonia oxidiser activity. For instance, there was no significant difference observed in soil pH (Table 1) and ammonia oxidiser abundance (Fig. 3a) between NP, NPK and 1/2NPK treatments, but the nitrification rate was the lowest in NP soil without K supply history, suggesting that increased K supply could be a factor enhancing the ammonia oxidiser activity. Additionally, the only soil without P fertiliser application (NK) had both the lowest ammonia oxidiser abundance and nitrification rate. Since NK soil also had the lowest pH, we cannot disentangle the influence of P element from the pH change. However, an enhanced microbial N cycling, including nitrification process, was previously observed following P addition in agricultural soils [43, 44]. Lastly, organic manure fertilisation history also had significant effect on the AOA composition (Table S1) and the soil receiving such fertilisation had the highest nitrification rate in our study (Fig. 3). Because this soil also had the highest pH, it is difficult to distinguish which factor is more determinant of such observation. Nevertheless, a previous study observed that organic mature fertilisation induced an increased nitrification rate without a change in soil pH [45], implying that other environmental elements important for ammonia oxidiser activity might have been introduced in soils following mature fertilisation regime. Indeed, the soil fertilised with manure accumulated the highest SOM content among all soils used in this study (Table 1) and such SOM concentration might favour high AOA growth, as AOA grow well under supply of ammonia originating from organic N mineralisation [22, 46].
Conclusion
The present study revealed a high abundance of Nitrososphaerales AOA in typical Chinese acidic red soils following different agricultural managements. Different long-term fertilisation regimes applied to the soils altered the relative abundance of several major Nitrososphaerales clades in soils, i.e. C1, C2 and C6, likely through perturbation of soil pH. Our results suggested that the high abundance and associated physiological adaptation to these low soil pH conditions were not restricted to previously recognized AOA clades, i.e. Ca. Nitrosotaleaceae C14 and Nitrososphaerales C11. The major Nitrososphaerales clades identified in our soils are potentially responsible for the in situ ammonia oxidation process, but assessment of their activity and relative contribution requires further investigation using high sensitivity techniques, such as DNA-stable isotope probing or RNA-based tools.
Data Availability
The pyrosequencing reads of archaeal amoA genes have been deposited at European Nucleotide Archive (ENA) with accession number PRJEB40021.
References
von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Tian D, Niu S (2015) A global analysis of soil acidification caused by nitrogen addition. Environ Res Lett 10. https://doi.org/10.1088/1748-9326/10/2/024019
Meng C, Tian D, Zeng H, Li Z, Yi C, Niu S (2019) Global soil acidification impacts on belowground processes. Environ Res Lett 14. https://doi.org/10.1088/1748-9326/ab239c
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75:139–157
Gubry-Rangin C, Hai B, Quince C, Engel M, Thomson BC, James P, Schloter M, Griffiths RI, Prosser JI, Nicol GW (2011) Niche specialization of terrestrial archaeal ammonia oxidizers. Proc Natl Acad Sci U S A 108:21206–21211. https://doi.org/10.1073/pnas.1109000108
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978. https://doi.org/10.1111/j.1462-2920.2008.01701.x
Gubry-Rangin C, Nicol GW, Prosser JI (2010) Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol 74:566–574. https://doi.org/10.1111/j.1574-6941.2010.00971.x
Lu L, Han W, Zhang J, Wu Y, Wang B, Lin X, Zhu J, Cai Z, Jia Z (2012) Nitrification of archaeal ammonia oxidizers in acid soils is supported by hydrolysis of urea. ISME J 6:1978–1984. https://doi.org/10.1038/ismej.2012.45
Zhang L-M, Hu H-W, Shen J-P, He J-Z (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6:1032–1045. https://doi.org/10.1038/ismej.2011.168
Xu X, Xia Z, Liu Y, Liu E, Muller K, Wang H, Luo J, Wu X, Beiyuan J, Fang Z, Xu J, Di H, Li Y (2021) Interactions between methanotrophs and ammonia oxidizers modulate the response of in situ methane emissions to simulated climate change and its legacy in an acidic soil. Sci Total Environ 752:142225. https://doi.org/10.1016/j.scitotenv.2020.142225
Zhang LM, Offre PR, He JZ, Verhamme DT, Nicol GW, Prosser JI (2010) Autotrophic ammonia oxidation by soil thaumarchaea. Proc Natl Acad Sci U S A 107:17240–17245. https://doi.org/10.1073/pnas.1004947107
Pratscher J, Dumont MG, Conrad R (2011) Ammonia oxidation coupled to CO2 fixation by archaea and bacteria in an agricultural soil. Proc Natl Acad Sci U S A 108:4170–4175. https://doi.org/10.1073/pnas.1010981108
Zhao J, Meng Y, Drewer J, Skiba UM, Prosser JI, Gubry-Rangin C (2020) Differential ecosystem function stability of ammonia-oxidizing archaea and bacteria following short-term environmental perturbation. mSystems 5. https://doi.org/10.1128/mSystems.00309-20
Aigle A, Gubry-Rangin C, Thion C, Estera-Molina KY, Richmond H, Pett-Ridge J, Firestone MK, Nicol GW, Prosser JI (2020) Experimental testing of hypotheses for temperature- and pH-based niche specialisation of ammonia oxidising archaea and bacteria. Environ Microbiol. https://doi.org/10.1111/1462-2920.15192
Aigle A, Prosser JI, Gubry-Rangin C (2019) The application of high-throughput sequencing technology to analysis of amoA phylogeny and environmental niche specialisation of terrestrial bacterial ammonia-oxidisers. Environ Microbiome 14.https://doi.org/10.1186/s40793-019-0342-6
Sheridan PO, Raguideau S, Quince C, Holden J, Zhang L, Thames C, Williams TA, Gubry-Rangin C (2020) Gene duplication drives genome expansion in a major lineage of Thaumarchaeota. Nat Commun 11:5494. https://doi.org/10.1038/s41467-020-19132-x
Alves RJE, Minh BQ, Urich T, von Haeseler A, Schleper C (2018) Unifying the global phylogeny and environmental distribution of ammonia-oxidising archaea based on amoA genes. Nat Commun 9:1517. https://doi.org/10.1038/s41467-018-03861-1
Gubry-Rangin C, Williams W, Prosser JI (2018) Approaches to understanding the ecology and evolution of understudied terrestrial archaeal ammonia-oxidisers. Emerg Top Life Sci 2:619–628. https://doi.org/10.1042/etls20180018
Wang B, Zheng Y, Huang R, Zhou X, Wang D, He Y, Jia Z (2014) Active ammonia oxidizers in an acidic soil are phylogenetically closely related to neutrophilic archaeon. Appl Environ Microbiol 80:1684–1691. https://doi.org/10.1128/AEM.03633-13
Pester M, Rattei T, Flechl S, Gröngröft A, Richter A, Overmann J, Reinhold-Hurek B, Loy A, Wagner M (2012) amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ Microbiol 14:525–539. https://doi.org/10.1111/j.1462-2920.2011.02666.x
Zhao J, Bello MO, Meng Y, Prosser JI, Gubry-Rangin C (2020) Selective inhibition of ammonia oxidising archaea by simvastatin stimulates growth of ammonia oxidising bacteria. Soil Biol Biochem. 141https://doi.org/10.1016/j.soilbio.2019.107673
Hink L, Gubry-Rangin C, Nicol GW, Prosser JI (2018) The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J 12:1084–1093. https://doi.org/10.1038/s41396-017-0025-5
Xu X, Liu Y, Singh BP, Yang Q, Zhang Q, Wang H, Xia Z, Di H, Singh BK, Xu J, Li Y (2020) NosZ clade II rather than clade I determine in situ N2O emissions with different fertilizer types under simulated climate change and its legacy. Soil Biol Biochem 150 https://doi.org/10.1016/j.soilbio.2020.107974
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di H (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374. https://doi.org/10.1111/j.1462-2920.2007.01358.x
Tourna M, Freitag TE, Nicol GW, Prosser JI (2008) Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 10:1357–1364. https://doi.org/10.1111/j.1462-2920.2007.01563.x
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102:14683–14688
Rotthauwe J-h, Witzel K-p, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Zhang J, Zhu T, Cai Z, Müller C (2011) Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil 342:419–432. https://doi.org/10.1007/s11104-010-0706-6
Norton JM, Alzerreca JJ, Suwa Y, Klotz MG (2002) Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch Microbiol 177:139–149. https://doi.org/10.1007/s00203-001-0369-z
Stieglmeier M, Mooshammer M, Kitzler B, Wanek W, Zechmeister-Boltenstern S, Richter A, Schleper C (2014) Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J 8:1135–1146. https://doi.org/10.1038/ismej.2013.220
Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW (2011) Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci 108:15892–15897. https://doi.org/10.1073/pnas.1107196108
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531. https://doi.org/10.1016/j.tim.2012.08.001
Philip D (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930
Gubry-Rangin C, Novotnik B, Mandič-Mulec I, Nicol GW, Prosser JI (2017) Temperature responses of soil ammonia-oxidising archaea depend on pH. Soil Biol Biochem 106:61–68. https://doi.org/10.1016/j.soilbio.2016.12.007
Liao J, Cao X, Zhao L, Wang J, Gao Z, Wang MC, Huang Y (2016) The importance of neutral and niche processes for bacterial community assembly differs between habitat generalists and specialists. FEMS Microbiol Ecol 92 https://doi.org/10.1093/femsec/fiw174
Sriswasdi S, Yang CC, Iwasaki W (2017) Generalist species drive microbial dispersion and evolution. Nat Commun 8:1162. https://doi.org/10.1038/s41467-017-01265-1
Jones SE, Lennon JT (2010) Dormancy contributes to the maintenance of microbial diversity. Proc Natl Acad Sci U S A 107:5881–5886. https://doi.org/10.1073/pnas.0912765107
Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci 105:11512–11519
Li Y, Xi R, Wang W, Yao H (2019) The relative contribution of nitrifiers to autotrophic nitrification across a pH-gradient in a vegetable cropped soil. J Soils Sediments 19:1416–1426. https://doi.org/10.1007/s11368-018-2109-x
Wang B, Qin W, Ren Y, Zhou X, Jung MY, Han P, Eloe-Fadrosh EA, Li M, Zheng Y, Lu L, Yan X, Ji J, Liu Y, Liu L, Heiner C, Hall R, Martens-Habbena W, Herbold CW, Rhee SK, Bartlett DH, Huang L, Ingalls AE, Wagner M, Stahl DA, Jia Z (2019) Expansion of Thaumarchaeota habitat range is correlated with horizontal transfer of ATPase operons. ISME J 13:3067–3079. https://doi.org/10.1038/s41396-019-0493-x
Herbold CW, Lehtovirta-Morley LE, Jung MY, Jehmlich N, Hausmann B, Han P, Loy A, Pester M, Sayavedra-Soto LA, Rhee SK, Prosser JI, Nicol GW, Wagner M, Gubry-Rangin C (2017) Ammonia-oxidising archaea living at low pH: insights from comparative genomics. Environ Microbiol 19:4939–4952. https://doi.org/10.1111/1462-2920.13971
Cheng Y, Wang J, Sun N, Xu M, Zhang J, Cai Z, Wang S (2018) Phosphorus addition enhances gross microbial N cycling in phosphorus-poor soils: a 15N study from two long-term fertilization experiments. Biol Fertil Soils 54:783–789. https://doi.org/10.1007/s00374-018-1294-5
Wei X, Hu Y, Peng P, Zhu Z, Atere CT, O’Donnell AG, Wu J, Ge T (2017) Effect of P stoichiometry on the abundance of nitrogen-cycle genes in phosphorus-limited paddy soil. Biol Fertil Soils 53:767–776. https://doi.org/10.1007/s00374-017-1221-1
Ouyang Y, Reeve JR, Norton JM (2018) Soil enzyme activities and abundance of microbial functional genes involved in nitrogen transformations in an organic farming system. Biol Fertil Soils 54:437–450. https://doi.org/10.1007/s00374-018-1272-y
Levicnik-Hofferle S, Nicol GW, Ausec L, Mandic-Mulec I, Prosser JI (2012) Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not added inorganic nitrogen. FEMS Microbiol Ecol 80:114–123. https://doi.org/10.1111/j.1574-6941.2011.01275.x
Acknowledgements
We thank Prof Yuanqiu He (now deceased) at the State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences for his contribution to this study. In addition, we thank the staff of the Analysis Center at the Institute of Soil Science for technical support, including Ms Rong Huang and Mr Zuohao Ma for 454-pyrosequencing, Mr Ruhai Wang for the ammonia and nitrate and nitrite content assays, Mr Guoxing Lu for the SOM assay. We also thank Dr Jian Cui and Dr Xiaoli Liu for assistance in soil sampling in the fields.
Funding
This work was supported by the National Natural Science Foundation of China (41530857, 91751204 and 41977056). JZ was funded by a Natural Environment Research Council grant (NE/K016342/1) and CGR was supported by a Royal Society University Research Fellowship (URF150571).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, J., Wang, B., Zhou, X. et al. Long-Term Adaptation of Acidophilic Archaeal Ammonia Oxidisers Following Different Soil Fertilisation Histories. Microb Ecol 83, 424–435 (2022). https://doi.org/10.1007/s00248-021-01763-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01763-2