Abstract
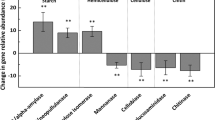
A resumption of climate warming in maritime Antarctica, arising from continued greenhouse gas emissions to the atmosphere, is predicted to lead to further expansions of plant populations across the region, with consequent increases in nutrient inputs to soils. Here, we test the main and interactive effects of warming, applied with open top chambers (OTCs), and nutrient amendment with tryptic soy broth (TSB), an artificial growth substrate, on bacterial community composition and diversity using Illumina sequencing of 16S rRNA genes in soil from a field experiment in the southern maritime Antarctic. Substantial effects of TSB application on bacterial communities were identified after 49 months, including reduced diversity, altered phylogenetic community assembly processes, increased Proteobacteria-to-Acidobacteria ratios and significant divergence in community composition, notably increases in the relative abundances of the gram-positive genera Arthrobacter, Paeniglutamicibacter and Planococcus. Contrary to previous observations from other maritime Antarctic field warming experiments, we recorded no effects of warming with OTCs, or interactive effects of OTCs and TSB application, on bacterial community composition or diversity. Based on these findings, we conclude that further warming of the maritime Antarctic is unlikely to influence soil bacterial community composition or diversity directly, but that increased nutrient inputs arising from enhanced plant growth across the region may affect the composition of soil bacterial communities, with possible effects on ecosystem productivity.



Similar content being viewed by others
References
Adams B et al (2009) The instrumental period. In: Turner J, Bindschadler R, Convey P, di Prisco G, Fahrbach E, Gutt J, Hodgson D, Mayewski P, Summerhayes C (eds) Antarctic Climate Change and the Environment. Scientific Committee on Antarctic Research, Scott Polar Research Institute, Cambridge, pp 183–298
Turner J, Lu H, White I, King JC, Phillips T, Hosking JS, Bracegirdle TJ, Marshall GJ, Mulvaney R, Deb P (2016) Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535:411–415
Bracegirdle TJ, Connolley WM, Turner J (2008) Antarctic climate change over the twenty first century. J Geophys Res 113:D03103
Bracegirdle TJ, Stephenson DB (2012) Higher precision estimates of regional polar warming by ensemble regression of climate model predictions. Clim Dyn 39:2805–2821
Vaughan DG, Marshall GJ, Connelley WM, Parkinson C, Mulvaney R, Hodgson DA, King JC, Pudsey CJ, Turner J (2003) Recent rapid regional climate warming on the Antarctic Peninsula. Clim Chang 60:243–274
Cook AJ, Fox AJ, Vaughan DG, Ferrigno JG (2005) Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science 308:541–544
Fowbert JA, Smith RIL (1994) Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct Alp Res 26:290–296
Royles J, Amesbury MJ, Convey P, Griffiths H, Hodgson DA, Leng MJ, Charman DJ (2013) Plants and soil microbes respond to recent warming on the Antarctic Peninsula. Curr Biol 23:1702–1706
Newsham KK, Hopkins DW, Carvalhais LC, Fretwell PT, Rushton SP, O’Donnell AG, Dennis PG (2016) Relationship between soil fungal diversity and temperature in the maritime Antarctic. Nat Clim Chang 6:182–186
Hill PW, Farrar J, Roberts P, Farrell M, Grant H, Newsham KK, Hopkins DW, Bardgett RD, Jones DL (2011) Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat Clim Chang 1:50–53
Hopkins DW, Sparrow AD, Shillam LL, English LC, Dennis PG, Novis P, Elberling B, Gregorich EG, Greenfield LG (2008) Enzymatic activities and microbial communities in an Antarctic dry valley soil: responses to C and N supplementation. Soil Biol Biochem 40:2130–2136
Dennis PG, Sparrow AD, Gregorich EG, Novis PM, Elberling B, Greenfield LG, Hopkins DW (2013) Microbial responses to carbon and nitrogen supplementation in an Antarctic dry valley soil. Antarct Sci 25:55–61
Dennis PG, Newsham KK, Rushton SP, Ord VJ, O’Donnell AG, Hopkins DW (2013) Warming constrains bacterial community responses to nutrient inputs in a southern, but not northern, maritime Antarctic soil. Soil Biol Biochem 57:248–255
Haack SD, Garchow H, Odelsen DA, Forney LJ, Klug MJ (1994) Accuracy, reproducibility, and interpretation of fatty acid methyl ester profiles of model bacterial communities. Appl Environ Microbiol 60:2483–2493
Yergeau E, Bokhorst S, Kang S, Zhou J, Greer CW, Aerts R, Kowalchuk GA (2012) Shifts in soil microorganisms in response to warming are consistent across a wide range of Antarctic environments. ISME J 6:692–702
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Wynn-Williams DD (1996) Response of pioneer soil microalgal colonists to environmental change in Antarctica. Microb Ecol 31:177–188
Sugden DE, Clapperton CM (1981) An ice-shelf moraine, George VI Sound, Antarctica. Ann Glaciol 2:135–141
Rinnan R, Michelsen A, Bååth E, Jonasson S (2007) Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem. Glob Chang Biol 13:28–39
Convey P, Smith RIL (1997) The terrestrial arthropod fauna and its habitats in northern Marguerite Bay and Alexander island, maritime Antarctic. Antarct Sci 9:12–26
Marion GM, Henry GHR, Freckman DW, Johnstone J, Jones G, Jones MH, Le Vesques E, Molau U, Mølgaard P, Parsons AN, Svoboda J, Virginia RA (1997) Open-top designs for manipulating field temperature in high-latitude ecosystems. Glob Chang Biol 3:20–32
Benhua S, Dennis PG, Laudicina VA, Ord VJ, Rushton SP, O’Donnell AG, Newsham KK, Hopkins DW (2014) Biogeochemical responses to nutrient, moisture, and temperature manipulations of soil from Signy Island in the maritime Antarctic. Antarct Sci 26:513–520
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1
Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD (2012) PANDAseq: paired-end assembler for illumina sequences. BMC Bioinform 13:31
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RI, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, vam Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Huse SM, Welch DM, Morrison HG, Sogin ML (2010) Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol 12:1889–1898
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Westcott SL, Schloss PD (2017) OptiClust, an improved method for assigning amplicon-based sequence data to operational taxonomic units. mSphere 2:e00073–e00017
Price MN, Dehal PS, Arkin AP (2010) FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
Stegen JC, Lin X, Konopka AE, Fredrickson JK (2012) Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J 6:1653–1664
Stegen JC, Lin X, Fredrickson JK, Chen X, Kennedy DW, Murray CJ, Rockhold ML, Konopka A (2013) Quantifying community assembly processes and identifying features that impose them. ISME J 7:2069–2079
Dini-Andreote F, Stegen JC, van Elsas JD, Salles JF (2015) Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc Natl Acad Sci U S A 112:E1326–E1332
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27:325–349
Clarke KR, Gorley RN (2006) PRIMERv6: User Manual/Tutorial. PRIMER-E, Plymouth
Winsley TJ, Snape I, McKinlay J, Stark J, van Dorst JM, Ji M, Ferrari BC, Siciliano SD (2014) The ecological controls on the prevalance of the candidate division TM7 in polar regions. Front Microbiol 5:345
Yergeau E, Bokhorst S, Huiskes AHL, Boschker HTS, Aerts R, Kowalchuk GA (2007) Size and structure of bacterial, fungal and nematode communities along an Antarctic environmental gradient. FEMS Microbiol Ecol 59:436–451
Christiansen CT, Haugwitz MS, Priemé A, Nielsen CS, Elberling B, Michelsen A, Grogan P, Blok D (2017) Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob Chang Biol 23:406–420
Sait M, Davis KER, Janssen PH (2006) Effect of pH on isolation and distribution of members of subdivision 1 of the phylum Acidobacteria occurring in soil. Appl Environ Microbiol 72:1852–1857
Yergeau E, Newsham KK, Pearce DA, Kowalchuk GA (2007) Patterns of bacterial diversity across a range of Antarctic terrestrial habitats. Environ Microbiol 9:2670–2682
Bokhorst S, Huiskes A, Convey P, Sinclair BJ, Lebouvier M, Van de Vijver B, Wall DH (2011) Microclimate impacts of passive warming methods in Antarctica: implications for climate change studies. Polar Biol 34:1421–1435
Deslippe JR, Hartmann M, Simard SW, Mohn WW (2012) Long-term warming alters the composition of Arctic soil microbial communities. FEMS Microbiol Ecol 82:303–315
Dorrepaal E, Toet S, Van Logtestijn RSP, Swart E, Van De Weg MJ, Callaghan TV, Aerts R (2009) Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460:616–619
Weedon JT, Kowalchuk GA, Aerts R, Freriks S, Röling WFM, van Bodegom PM (2017) Compositional stability of the bacterial community in a climate-sensitive sub-Arctic peatland. Front Microbiol 8:317
Radujković D, Verbruggen E, Sigurdsson BD, Leblans NIW, Janssens IA, Vicca S, Weedon JT (2018) Prolonged exposure does not increase soil microbial community compositional response to warming along geothermal gradients. FEMS Microbiol Ecol 94. https://doi.org/10.1093/femsec/fix174
Bockheim JG, Schaefer CEGR (2015) Soils of Ellsworth Land, the Ellsworth Mountains. In: Bockheim JG (ed) The soils of Antarctica. World Soils Book Series. Springer, Switzerland, pp 169–181
Bokhorst S, Huiskes A, Convey P, van Bodegom PM, Aerts R (2008) Climate change effects on soil arthropod communities from the Falkland Islands and the maritime Antarctic. Soil Biol Biochem 40:1547–1556
Dennis PG, Newsham KK, Rushton SP, O’Donnell AG, Hopkins DW (2019) Soil bacterial diversity is positively associated with air temperature in the maritime Antarctic. Sci Rep 9:2686. https://doi.org/10.1038/s41598-019-39521-7
Chong CW, Silvaraj S, Supramanian Y, Snape I, Tan IKP (2018) Effect of temperature on bacterial community in petroleum hydrocarbon-contaminated and uncontaminated Antarctic soil. Polar Biol 41:1763–1775
Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin III FS (2004) Ecosystem carbon storage in Arctic tundra reduced by long-term nutrient fertilization. Nature 431:440–443
Campbell BJ, Polson SW, Hanson TE, Mack MC, Schuur EAG (2010) The effect of nutrient deposition on bacterial communities in Arctic tundra soil. Environ Microbiol 12:1842–1854
Koyama A, Wallenstein MD, Simpson RT, Moore JC (2014) Soil bacterial community composition altered by increased nutrient availability in Arctic tundra soils. Front Microbiol 5:516
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annu Rev Ecol Syst 33:475–505
Newsham KK, Pearce DA, Bridge PD (2010) Minimal influence of water and nutrient content on the bacterial community composition of a maritime Antarctic soil. Microbiol Res 165:523–530
Chong CW, Convey P, Pearce DA, Tan IKP (2012) Assessment of soil bacterial communities on Alexander Island (in the maritime and continental Antarctic transitional zone). Polar Biol 35:387–399
Shaver G (2005) Daily summary of 10 cm soil temperatures in the Arctic LTER moist acidic experimental plots from 1998 to present, Toolik Lake Field Station, Alaska. Environ Data Initiative. https://doi.org/10.6073/pasta/89b6208bc6631129949eeca791063ed3 Accessed 12 September 2018
Smith JJ, Tow LA, Stafford W, Cary C, Cowan DA (2006) Bacterial diversity in three different Antarctic cold desert mineral soils. Microb Ecol 51:413–421
Babalola O, Kirby BM, Le Roes-Hill M, Cook AE, Cary SC, Burton SG, Cowan DA (2009) Phylogenetic analysis of actinobacterial populations associated with Antarctic Dry Valley mineral soils. Environ Microbiol 11:566–576
Costello EK, Halloy SRP, Reed SC, Sowell P, Schmidt SK (2009) Fumarole-supported islands of biodiversity within a hyperarid, high-elevation landscape on Socompa Volcano, Puna de Atacama, Andes. Appl Environ Microbiol 75:735–747
Solon AJ, Vimercati L, Darcy JL, Arán P, Porazinska D, Dorador C, Farías ME, Schmidt SK (2018) Microbial communities of high-elevation fumaroles, Penitentes, and dry tephra “soils” of the Puna de Atacama volcanic zone. Microb Ecol 76:340–351
Acknowledgements
Logistical support was provided by the British Antarctic Survey’s Operations Unit, with Air Unit pilots Alan Meredith, Steve King, Doug Pearson and Ian Potten providing access to Mars Oasis. Adam Clark, Dickie Hall, Sharon Duggan and Paul Dennis provided valuable support. Two anonymous reviewers provided helpful comments. All are gratefully acknowledged.
Funding
This research was funded by the British Antarctic Survey’s Long-Term Monitoring and Survey programme, the NERC Antarctic Funding Initiative (grant number NE/D00893X/1) and a National Research Foundation of Korea Grant from the Korean Government (grant number 2018R1C1B6007755).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(PDF 246 kb)
Rights and permissions
About this article
Cite this article
Newsham, K.K., Tripathi, B.M., Dong, K. et al. Bacterial Community Composition and Diversity Respond to Nutrient Amendment but Not Warming in a Maritime Antarctic Soil. Microb Ecol 78, 974–984 (2019). https://doi.org/10.1007/s00248-019-01373-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01373-z