Abstract
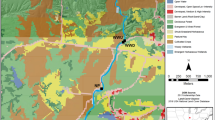
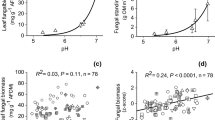
In the Arctic, climate changes contribute to enhanced mobilization of organic matter in streams. Microbial extracellular enzymes are important mediators of stream organic matter processing, but limited information is available on enzyme processes in this remote area. Here, we studied the variability of microbial extracellular enzyme activity in high-Arctic fluvial biofilms. We evaluated 12 stream reaches in Northeast Greenland draining areas exhibiting different geomorphological features with contrasting contents of soil organic matter to cover a wide range of environmental conditions. We determined stream nitrogen, phosphorus, and dissolved organic carbon concentrations, quantified algal biomass and bacterial density, and characterized the extracellular enzyme activities involved in catalyzing the cleavage of a range of organic matter compounds (e.g., β-glucosidase, phosphatase, β-xylosidase, cellobiohydrolase, and phenol oxidase). We found significant differences in microbial organic matter utilization among the study streams draining contrasting geomorphological features, indicating a strong coupling between terrestrial and stream ecosystems. Phosphatase and phenol oxidase activities were higher in solifluction areas than in alluvial areas. Besides dissolved organic carbon, nitrogen availability was the main driver controlling enzyme activities in the high-Arctic, which suggests enhanced organic matter mineralization at increased nutrient availability. Overall, our study provides novel information on the controls of organic matter usage by high-Arctic stream biofilms, which is of high relevance due to the predicted increase of nutrient availability in high-Arctic streams in global climate change scenarios.





Similar content being viewed by others
References
Tarnocai C, Canadell JG, Schuur EAG et al (2009) Soil organic carbon pools in the northern circumpolar permafrost region. Glob Biogeochem Cycles 23:1–11. https://doi.org/10.1029/2008GB003327
Panneer Selvam B, Lapierre J-F, Guillemette F et al (2017) Degradation potentials of dissolved organic carbon (DOC) from thawed permafrost peat. Sci Rep 7:45811. https://doi.org/10.1038/srep45811
Drake TW, Wickland KP, Spencer RGM et al (2015) Ancient low-molecular-weight organic acids in permafrost fuel rapid carbon dioxide production upon thaw. PNAS 112:13946–11395. https://doi.org/10.1073/pnas.1511705112
Elberling B, Michelsen A, Schädel C et al (2013) Long-term CO2 production following permafrost thaw. Nat Clim Chang 3:890–894. https://doi.org/10.1038/nclimate1955
Abbott BW, Larouche JR, Jones JB, Bowden WB, Balser AW (2014) Elevated dissolved organic carbon biodegradability from thawing and collapsing permafrost. J Geophys Res Biogeosciences 119(10):2049–2063
Crowther T, Todd-Brown K, Rowe C et al (2016) Quantifying global soil C losses in response to warming. Nature 540:104–108. https://doi.org/10.1038/nature20150
Frey KE, McClelland JW (2009) Impacts of permafrost degradation on arctic river biogeochemistry. Hydrol Process 23:169–182. https://doi.org/10.1002/hyp
Bowden WB, Gooseff MN, Balser A et al (2008) Sediment and nutrient delivery from thermokarst features in the foothills of the North Slope, Alaska: potential impacts on headwater stream ecosystems. J Geophys Res Biogeosci 113:1–12. https://doi.org/10.1029/2007JG000470
Olefeldt D, Goswami S, Grosse G et al (2016) Circumpolar distribution and carbon storage of thermokarst landscapes. Nat Commun 7:1–11. https://doi.org/10.1038/ncomms13043
Vonk JE, Tank SE, Bowden WB et al (2015) Reviews and syntheses: effects of permafrost thaw on Arctic aquatic ecosystems. Biogeosciences 12:7129–7167. https://doi.org/10.5194/bg-12-7129-2015
Anderson NJ, Saros JE, Bullard JE et al (2017) The Arctic in the twenty-first century: changing biogeochemical linkages across a paraglacial landscape of Greenland. Bioscience 67:biw158. https://doi.org/10.1093/biosci/biw158
Cole JJ, Prairie YT, Caraco NF et al (2007) Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10:172–185
Battin TJ, Kaplan LA, Findlay S et al (2009) Biophysical controls on organic carbon fluxes in fluvial networks. Nat Geosci 2:595–595. https://doi.org/10.1038/ngeo602
Mann PJ, Eglinton TI, Mcintyre CP et al (2015) Utilization of ancient permafrost carbon in headwaters of Arctic fluvial networks OPEN. Nat Commun 6:1–7. https://doi.org/10.1038/ncomms8856
Spencer RGM, Man PJ, Dittmar T et al (2015) Detecting the signature of permafrost thaw in Arctic rivers. Geophys Res Lett 42:2830–2835. https://doi.org/10.1002/2015GL063498.Received
Arnosti C (2003) Microbial extracellular enzymes and their role in dissolved organic matter cycling. In: Findlay SEG, Sinsabaugh RL (eds) Aquatic ecosystems interactivity of dissolved organic matter. Academic Press, Elsevier
Battin TJ, Besemer K, Bengtsson MM et al (2016) The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol 14:251–263. https://doi.org/10.1038/nrmicro.2016.15
Sinsabaugh RL, Osgood MP, Findlay S (1994) Enzymatic models for estimating decomposition rates of particulate detritus. J North Am Benthol Soc 13:160–169
Steen AD, Arnosti C (2013) Extracellular peptidase and carbohydrate hydrolase activities in an Arctic fjord (Smeerenburgfjord, Svalbard). Aquat Microb Ecol 69:93–99. https://doi.org/10.3354/ame01625
Steen AD, Arnosti C (2011) Long lifetimes of B-glucosidase, leucine aminopeptidase, and phosphatase in Arctic seawater. Mar Chem 123:127–132. https://doi.org/10.1016/j.marchem.2010.10.006
Arnosti C, Steen AD, Ziervogel K et al (2011) Latitudinal gradients in degradation of marine dissolved organic carbon. PLoS One 6:8–13. https://doi.org/10.1371/journal.pone.0028900
Mann PJ, Sobczak WV, Larue MM et al (2014) Evidence for key enzymatic controls on metabolism of Arctic river organic matter. Glob Chang Biol 20:1089–1100. https://doi.org/10.1111/gcb.12416
Freimann R, Bu H, Findlay SEG, Robinson CT (2013) Response of lotic microbial communities to altered water source and nutritional state in a glaciated alpine floodplain. Limnol Oceanogr 58:951–965. https://doi.org/10.4319/lo.2013.58.3.0951
Freimann R, Bürgmann H, Findlay SE, Robinson CT (2013) Bacterial structures and ecosystem functions in glaciated floodplains: contemporary states and potential future shifts. ISME J 7:2361–2373. https://doi.org/10.1038/ismej.2013.114
Catalán N, Marcé R, Kothawala DN, Tranvik LJ (2016) Organic carbon decomposition rates controlled by water retention time across inland waters. Nat Geosci 9:501–504. https://doi.org/10.1038/ngeo2720
Romaní AM, Artigas J, Ylla I (2012) Extracellular enzymes in aquatic biofilms: microbial interactions versus water quality effects in the use of organic matter. In: Lear G, Lewis GD (eds) Microbial biofilms: current research and applications. Caister Academic Press, pp 153–174
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–343. https://doi.org/10.1146/annurev-ecolsys-071112-124414
Harms TK, Abbott BW, Jones JB (2014) Thermo-erosion gullies increase nitrogen available for hydrologic export. Biogeochemistry 117:299–311. https://doi.org/10.1007/s10533-013-9862-0
Lafrenière M, Louiseize N, Lamoureux S (2017) Active layer slope disturbances affect seasonality and composition of dissolved nitrogen export from High Arctic headwater catchments. Arct Sci 450:429–450
Docherty CL, Riis T;, Hannah D;, et al (2018) Nutrient uptake controls and limitation dynamics in northeast Greenland streams. Polar Res. 37. https://doi.org/10.1080/17518369.2018.1440107
Shelef E, Rowland JC, Wilson CJ et al (2017) Large uncertainty in permafrost carbon stocks due to hillslope soil deposits. Geophys Res Lett 44(12):6134–6144
Cable S, Christiansen HH, Westergaard-Nielsen A et al (2017) Geomorphological and cryostratigraphical analyses of the Zackenberg Valley, NE Greenland and significance of Holocene alluvial fans. Geomorphology 303:504–523. https://doi.org/10.1016/j.geomorph.2017.11.003
Michaelson GJ, Ping CL, Kimble JM (1996) Carbon storage and distribution in tundra soils of Arctic Alaska, U.S.A. Arct Alp Res 28:414–424. https://doi.org/10.1107/S0567740869002214
Hawkings J, Wadham J, Tranter M et al (2016) The Greenland Ice Sheet as a hot spot of phosphorus weathering and export in the Arctic. Glob Biogeochem Cycles 30:191–210. https://doi.org/10.1002/2015GB005237.Received
Blaen PJ, Hannah DM, Brown LE, Milner AM (2014) Water source dynamics of high Arctic river basins. Hydrol Process 28:3521–3538. https://doi.org/10.1002/hyp.9891
Christiansen HH, Sigsgaard C, Humlum O et al (2008) Permafrost and periglacial geomorphology at Zackenberg. Adv Ecol Res 40:151–174. https://doi.org/10.1016/S0065-2504(07)00007-4
Hasholt B, Hagedorn B (2000) Hydrology and geochemistry of river-borne material in a high Arctic drainage system, Zackenberg, Northeast Greenland. Arct Antarct Alp Res 32:84–94
Elberling BO, Tamstorf MP, Michelsen A, et al (2008) Soil and plant community—characteristics and dynamics at Zackenberg. In: Advances in ecological research. Elsevier Ltd, pp 223–2448
Palmtag J, Cable S, Christiansen HH et al (2018) Landform partitioning and estimates of deep storage of soil organic matter in Zackenberg, Greenland. Cryopshere 12:1735–1744
Helms JR, Stubbins A, Ritchie JD et al (2008) Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr 53:955–969. https://doi.org/10.4319/lo.2008.53.3.0955
Weishaar JL, Aiken GR, Bergamaschi BA et al (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708. https://doi.org/10.1021/es030360x
Green SA, Blough NV (1994) Optical absorption and fluorescence properties of chromophoric dissolved organic matter in natural waters. Limnol Oceanogr 39:1903–1916. https://doi.org/10.4319/lo.1994.39.8.1903
Poulin BA, Ryan JN, Aiken GR (2014) Effects of iron on optical properties of dissolved organic matter. Environ Sci Technol 48:10098–10106. https://doi.org/10.1021/es502670r
Steinman AD, Lamberti GA, Leavitt PR (2006) Biomass and pigments of benthic algae. In: Lamberti FR, Hauer GA (eds) Methods in stream ecology. Academic Press, San Diego, pp 357–380
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798. https://doi.org/10.1038/nature08632
Ylla I, Peter H, Romaní AM, Tranvik LJ (2013) Different diversity-functioning relationship in lake and stream bacterial communities. FEMS Microbiol Ecol 85:95–103. https://doi.org/10.1111/1574-6941.12101
Romaní AM, Sabater S (2000) Influence of algal biomass on extracellular enzyme activity in river biofilms. Microb Ecol 41:16–24. https://doi.org/10.1007/s002480000041
Sinsabaugh RL, Follstad JJ (2011) Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry 102:31–43. https://doi.org/10.1007/s10533-010-9482-x
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. A practical information-theoretic approach, 2nd ed. Springer-Verlag, New york
Calcagno V, de Mazancourt C (2010) Glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Softw 34(12):1–29
Grömping U (2006) Relative importance for linear regression in R: the package relaimpo. J Stat Softwater 17(1):1–27
Muggeo VMR (2008) segmented: an R package to fit regression models with broken-line relationships. R NEWS 8:120–125
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Palmtag J, Hugelius G, Lashchinskiy N et al (2015) Storage, landscape distribution, and burial history of soil organic matter in contrasting areas of continuous permafrost. Arct Antarct Alp Res 47:71–88. https://doi.org/10.1657/AAAR0014-027
Freeman C, Ostle N, Kang H (2001) An enzymic “latch” on a global carbon store. Nature 409:149. https://doi.org/10.1038/35051650
Kawahigashi M, Kaiser K, Kalbitz K et al (2004) Dissolved organic matter in small streams along a gradient from discontinuous to continuous permafrost. Glob Chang Biol 10:1576–1586. https://doi.org/10.1111/j.1365-2486.2004.08827.x
Rier ST, Shirvinski JM, Kinek KC (2014) In situ light and phosphorus manipulations reveal potential role of biofilm algae in enhancing enzyme-mediated decomposition of organic matter in streams. Freshw Biol 59:1039–1051. https://doi.org/10.1111/fwb.12327
Cory RM, Ward CP, Crump BC, Kling GW (2014) Sunlight controls water column processing of carbon in arctic fresh waters. Science(80) 345:925–928. https://doi.org/10.1126/science.1253119
Palmtag J, Cable S, Christiansen HH et al (2017) Improved landscape partitioning and estimates of deep storage of soil organic carbon in the Zackenberg area (NE Greenland) using geomorphological landforms. Cryosph Discuss In Review. https://doi.org/10.5194/tc-2017-255
Tank SE, Fellman JB, Hood E, Kritzberg ES (2018) Beyond respiration: controls on lateral carbon fluxes across the terrestrial-aquatic interface. Limnol Oceanogr Lett. https://doi.org/10.1002/lol2.10065
Craine JM, Brookshire ENJ, Cramer MD et al (2015) Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 396:1–26. https://doi.org/10.1007/s11104-015-2542-1
Christian JR, Karl DM (1995) Bacterial ectoenzymes in marine waters: activity ratios and temperature responses in three oceanographic provinces. Limnol Oceanogr 40:1042–1049. https://doi.org/10.4319/lo.1995.40.6.1042
Margalef O, Sardans J, Fernández-Martínez M et al (2017) Global patterns of phosphatase activity in natural soils. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-01418-8
Wallenstein MD, Mcmahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Chang Biol 15:1631–1639. https://doi.org/10.1111/j.1365-2486.2008.01819.x
Ylla I, Romaní AM, Sabater S (2012) Labile and recalcitrant organic matter utilization by river biofilm under increasing water temperature. Microb Ecol 64:593–604. https://doi.org/10.1007/s00248-012-0062-6
Frossard A, Gerull L, Mutz M, Gessner MO (2013) Litter supply as a driver of microbial activity and community structure on decomposing leaves: a test in experimental streams. Appl Environ Microbiol 79:4965–4973. https://doi.org/10.1128/AEM.00747-13
Mulholland PJ, Rosemond A (1992) Periphyton response to longitudinal nutrient depletion in a woodland stream: evidence of upstream-downstream. J North Am Benthol Soc 11:405–419
Amon RMW, Rinehart AJ, Duan S et al (2012) Dissolved organic matter sources in large Arctic rivers. Geochim Cosmochim Acta 94:217–237. https://doi.org/10.1016/j.gca.2012.07.015
Wauthy M, Rautio M, Christoffersen KS et al (2018) Increasing dominance of terrigenous organic matter in circumpolar freshwaters due to permafrost thaw. Limnol Oceanogr Lett 3:186–198. https://doi.org/10.1002/lol2.10063
Romaní AM, Vázquez E, Butturini A (2006) Microbial availability and size fractionation of dissolved organic carbon after drought in an intermittent stream: biogeochemical link across the stream-riparian interface. Microb Ecol 52:501–512. https://doi.org/10.1007/s00248-006-9112-2
Sinsabaugh RL, Gallo ME, Lauber C et al (2005) Extracellular enzyme activities and soil organic matter dynamics for Northern Hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75:201–215
Myrstener M, Rocher-Ros G, Burrows RM et al (2018) Persistent nitrogen limitation of stream biofilm communities along climate gradients in the arctic. Glob Chang Biol 24:3680–3691
Houlton BZ, Dahlgren RA (2018) Convergent evidence for widespread rock nitrogen sources in Earth’s surface environment. Science (80-) 62:58–62
Ylla I, Borrego CM, Romaní AM, Sabater S (2009) Availability of glucose and light modulates the structure and function of a microbial biofilm. FEMS Microbiol Ecol 69:27–42. https://doi.org/10.1111/j.1574-6941.2009.00689.x
Chróst RJ (1991) Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In: Chróst RJ (ed) Microbial enzymes in aquatic environments. Brock/Springer, New York, pp 29–59
Whitton B (1991) Use of phosphatase assays with algae to assess phosphorous status of aquatic environments. In: Jeffrey D, Madden B (eds) Bioindicators and environmental management. Academic Press, London, pp 295–310
Chrost RJ (1986) Algal-bacterial metabolic coupling in the carbon and phosphorus cycle in lakes. In: Meguar F, Gantar M (eds) Perspectives in microbial ecology. Slovene Society for Microbiology, pp 360–366
Walker MD, Wahren CH, Hollister RD et al (2006) Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci U S A 103:1342–1346. https://doi.org/10.1073/pnas.0503198103
Nabe-Nielsen J, Normand S, Hui FKC et al (2017) Plant community composition and diversity in the high arctic tundra: from the present to the future. Ecol Evol 7:1–10. https://doi.org/10.1002/ece3.3496
Freeman C, Evans CD, Monteith DT et al (2001) Export of organic carbon from peat soils. Nature 412:785–785. https://doi.org/10.1038/35090628
Ylla I, Canhoto C, Romaní AM (2014) Effects of warming on stream biofilm organic matter use capabilities. Microb Ecol 68:132–145. https://doi.org/10.1007/s00248-014-0406-5
Holtgrieve GW, Schindler DE, Hobbs WO et al (2011) A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the Northern Hemisphere. Science (80-) 334:1545–1548. https://doi.org/10.1126/science.1212267
Kühnel R, Roberts TJ, Björkman MP et al (2011) 20-Year climatology of NO3- and NH4+ wet deposition at Ny-Ålesund, Svalvard. Adv Meteorol 2011:1–10. https://doi.org/10.1155/2011/406508
Tye AM, Heaton THE (2007) Chemical and isotopic characteristics of weathering and nitrogen release in non-glacial drainage waters on Arctic tundra. Geochim Cosmochim Acta 71:4188–4205. https://doi.org/10.1016/j.gca.2007.06.040
Louiseize NL, Lafrenière MJ, Hastings MG (2014) Stable isotopic evidence of enhanced export of microbially derived NO-3 following active layer slope disturbance in the Canadian High Arctic. Biogeochemistry 121:565–580. https://doi.org/10.1007/s10533-014-0023-x
Harms TK, Jones JB (2012) Thaw depth determines reaction and transport of inorganic nitrogen in valley bottom permafrost soils. Glob Chang Biol 18:2958–2968. https://doi.org/10.1111/j.1365-2486.2012.02731.x
Lamoureux SF, Lafreniére MJ (2009) Fluvial impact of extensive active layer detachments, Cape Bounty , Melville Island. Arct Antarct Alp Res 41:59–68
Docherty CL, Hannah DM, Riis T et al (2017) Large thermo-erosional tunnel for a river in northeast Greenland. Polar Sci 14:1–5. https://doi.org/10.1016/j.polar.2017.08.001
MacLean R, Oswood MW, Irons JG, McDowell WH (1999) The effect of permafrost on stream biogeochemistry: a case study of two streams in the Alaskan (U.S.A.) taiga. Biogeochemistry 47:239–267. https://doi.org/10.1007/BF00992909
Acknowledgements
The authors thank Biobasis, Geobasis, and Zackenberg logistics for assistance at the Zackenberg Research Station. We are grateful to the GeoBasis Programme of the Zackenberg research station and Stefanie Cable for providing DEM and geomorphological data, respectively. Special thanks to Lone J. Ottosen, Malin Camilla Håckansson, and Birgitte Kretzschmar Tagesen for their support in laboratory analysis.
Funding
This work was financially supported by the Carlsberg Foundation (grant number CF16-0325). AP has received additional support from the Ramón Areces Foundation postgraduate studies program.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 377 kb)
Rights and permissions
About this article
Cite this article
Pastor, A., Freixa, A., Skovsholt, L.J. et al. Microbial Organic Matter Utilization in High-Arctic Streams: Key Enzymatic Controls. Microb Ecol 78, 539–554 (2019). https://doi.org/10.1007/s00248-019-01330-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01330-w