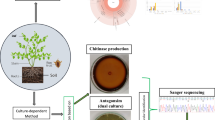
Abstract
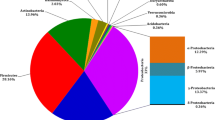
Sap-feeding insects harbor diverse microbial endosymbionts that play important roles in host ecology and evolution, including contributing to host pest status. The vine mealybug, Planococcus ficus, is a serious pest of grapevines, vectoring a number of pathogenic grape viruses. Previous studies have shown that virus transmission is abolished when mealybugs are raised in the laboratory on potato. To examine the possible role of microbial symbionts in virus transmission, the archaeal, bacterial, and fungal microbiota of field and laboratory P. ficus were characterized using molecular and classical microbiological methods. Lab and field colonies of P. ficus harbored different microbiota. While both were dominated by the bacterial obligate nutritional symbionts Moranella and Tremblaya, field samples also harbored a third bacterium that was allied with cluster L, a lineage of bacterial symbionts previously identified in aphids. Archaea were not found in any of the samples. Fungal communities in field-collected mealybugs were dominated by Metschnikowia and Cladosporium species, while those from laboratory-reared mealybugs were dominated by Alternaria and Cladosporium species. In conclusion, this study has identified a diverse set of microbes, most of which appear to be facultatively associated with P. ficus, depending on environmental conditions. The role of various members of the mealybug microbiome, as well as how the host plant affects microbial community structure, remains to be determined.





Similar content being viewed by others
References
Assaf LH, Haleem RA, Abdullah SK (2011) Association of entomopathogenic and other opportunistic fungi with insects in dormant locations. Jordan J Biol Sci 4:87–92
Ben-Dov Y (1994) A systematic catalogue of the mealybugs of the world (Insecta: Homoptera: Coccoidea: Pseudococcidae and Putoidae) with data on geographical distribution, host plants, biology and economic importance. Intercept Ltd., Andover
Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Crous PW (2010) Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud Mycol 67:1–94
Brady CM, White JA (2013) Cowpea aphid (Aphis craccivora) associated with different host plants has different facultative endosymbionts. Ecol Entomol 38:433–437
Bressan A, Arneodo JD, Simonato M, Haines WP, Boudon-Padieu E (2009) Characterization and evolution of two bacteriome-inhabiting symbionts in cixiid planthoppers (Hemiptera: Fulgoromorpha: Pentastirini). Environ Microbiol 11:3265–3279
Buchner P (1965) Endosymbiosis of animals with plant microorganisms. Interscience, New York
Burke GR, Normark BB, Favret C, Moran NA (2009) Evolution and diversity of facultative symbionts from the aphid subfamily Lachninae. Appl Environ Microbiol 75:5328–5335
Cariveau DP, Powell JE, Koch H, Winfree R, Moran NA (2014) Variation in gut microbial communities and its association with pathogen infection in wild bumble bees (Bombus). ISME J. doi:10.1038/ismej.2014.68
Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A (2011) Bacterial communities of diverse Drosophila species: ecological context of a host–microbe model system. PLoS Genet 7:e1002272
Charles JG (1982) Economic damage and preliminary thresholds for mealybugs in Auckland vineyards. N Z J Agric Res 25:415–420
Chen H, Rangasamy M, Tan SY, Wang H, Siegfried BD (2010) Evaluation of five methods for total DNA extraction from western corn rootworm beetles. PLoS ONE 5:e11963
Christias CH, Hatzipapas P, Dara A, Kaliafas A, Chrysanthis G (2001) Alternaria alternata, a new pathotype pathogenic to aphids. BioControl 46:105–124
Dellavalle PD, Cabrera A, Alem D, Larrañaga P, Ferreira F, Rizza MD (2011) Antifungal activity of medicinal plant extracts against phytopathogenic fungus Alternaria spp. Chil J Agric Res 71:231–239
Dillon RJ, Dillon VM (2004) The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49:71–92
Dong SZ, Pang K, Bai X, Yu XP, Hao PY (2011) Identification of two species of yeast-like symbiotes in the brown planthopper, Nilaparvata lugens. Curr Microbiol 62:1133–1138
Dowd SE, Sun Y, Wolcott RD, Domingo A, Carroll JA (2008) Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: bacterial diversity in the ileum of newly weaned Salmonella infected pigs. Foodborne Pathog Dis 5:459–472
Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Heled J, Kearse M, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A (2010) Geneious v5.1. Available from: URL http://www.geneious.com
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinforma 5:113
Eken C, Hayat R (2009) Preliminary evaluation of Cladosporium cladosporioides (Fresen.) de Vries in laboratory conditions, as a potential candidate for biocontrol of Tetranychus urticae Koch. World J Microbiol Biotechnol 25:489–492
Engelbrecht DJ, Kasdorf GGF (1990) Transmission of grapevine leafroll disease and associated closteroviruses by the vine mealybug Planococcus ficus. Phytophylactica 22:341–346
Franke-Whittle IH, O’Shea MG, Leonard GJ, Sly LI (2004) Molecular investigation of the microbial populations of the pink sugarcane mealybug, Saccharicoccus sacchari. Ann Microbiol 54:455–470
Frohlich T-JI, Bedford I, Markham P, Brown J (1999) A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol Ecol 8:1683–1691
Fukatsu T, Ishikawa H (1996) Phylogenetic position of yeast-like symbiont of Hamiltonaphis styraci (Homoptera, Aphididae) based on 18S rDNA sequence. Insect Biochem Mol Biol 4:383–388
Fukatsu T, Nikoh N (2000) Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawli (Insecta, Homoptera). Appl Environ Microbiol 66:643–650
Gibson CM, Hunter MS (2010) Extraordinarily widespread and fantastically complex: comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol Lett 13:223–234
Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, Tzuri GA, Horowitz R, Belausov E, Mozes-Daube N, Kontsedalov K, Gershon M, Gal S, Katzir N, Zchori-Fein E (2006) Identification and localization of a Rickettsia sp. in Bemisia tabaci (Hemiptera: Aleyrodidae). Appl Environ Microbiol 72:3646–3652
Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Kontsedalov S, Skaljac M, Brumin M, Sobol I, Czosnek H, Vavre F, Fleury F, Ghanim M (2010) The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J Virol 84:9310–9317
Hammer O, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hara K, Shinzato N, Seo M, Oshima T, Yamagishi A (2002) Phylogenetic analysis of symbiotic archaea living in the gut of xylophagous cockroaches. Microbes Environ 17:185–190
Henry LM, Peccoud J, Simon JC, Hadfield JD, Maiden MJ, Ferrari J, Godfray HCJ (2013) Horizontally transmitted symbionts and host colonization of ecological niches. Curr Biol 23:1713–1717
Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T (2007) Obligate symbiont involved in pest status of host insect. Proc R Soc B Biol Sci 274:1979–1984
Hughes GL, Allsopp PG, Webb RI, Yamada R, Iturbe-Ormaetxe I, Brumbley SM, O’Neill SL (2011) Identification of yeast associated with the planthopper, Perkinsiella saccharicida: potential applications for Fiji leaf gall control. Curr Microbiol 63:392–401
Iasur-Kruh L, Weintraub PG, Mozes-Daube N, Robinson WE, Perlman SJ, Zchori-Fein E (2013) Novel Rickettsiella bacterium in the leafhopper Orosius albicinctus (Hemiptera: Cicadellidae). Appl Environ Microbiol 79:4246–4252
Kikuchi Y, Hosokawa T, Fukatsu T (2011) Specific developmental window for establishment of an insect-microbe gut symbiosis. Appl Environ Microbiol 77:4075–4081
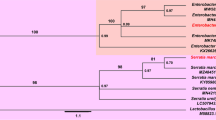
Koga R, Nikoh N, Matsuura Y, Meng XY, Fukatsu T (2012) Mealybugs with distinct endosymbiotic systems living on the same host plant. FEMS Microbiol Ecol 83:93–100
Kwong WK, Moran NA (2013) Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. Int J Syst Evol Microbiol 63:2008–2018
Lamelas A, Pérez-Brocal V, Gómez-Valero L, Gosalbes MJ, Moya A, Latorre A (2008) Evolution of the secondary symbiont “Candidatus Serratia symbiotica” in aphid species of the subfamily Lachninae. Appl Environ Microbiol 74:4236–4240
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Malakar-Kuenen R, Daane KM, Bentley W, Yokota GY, Martin L, Godfrey K, Ball J (2001). Population dynamics of the vine mealybug and its natural enemies in the Coachella and San Joaquin Valleys. University of California, Kearney Plant Protection Group, Plant Protection Quarterly 11:1–5
Mansour R, Suma P, Mazzeo G, La Pergola A, Pappalardo V, Lebdi KG, Russo A (2012) Interactions between the ant Tapinoma nigerrimum (Hymenoptera: Formicidae) and the main natural enemies of the vine and citrus mealybugs (Hemiptera: Pseudococcidae). Biocontrol Sci Tech 22:527–537
Martinson VG, Moy J, Moran NA (2012) Establishment of characteristic gut bacteria during development of the honeybee worker. Appl Environ Microbiol 78:2830–2840
Minafra A, Hadidi A (1994) Sensitive detection of grapevine virus A, B, or leafroll-associated virus III from viruliferous mealybugs and infected tissue by cDNA amplification. J Virol Methods 47:175–188
Montllor CB, Maxmen A, Purcell AH (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol 27:189–195
Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190
Moran NA, Tran P, Gerardo NM (2005) Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl Environ Microbiol 71:8802–8810
Morin S, Ghanim M, Sobol I, Czosnek H (2000) The GroEL protein of the whitefly Bemisia tabaci interacts with the coat protein of transmissible and nontransmissible begomoviruses in the yeast two-hybrid system. Virology 276:404–416
Munson MA, Baumann P, Kinsey MG (1991) Buchnera gen. nov. and Buchnera aphidicola sp. nov., a taxon consisting of the mycetocyte-associated, primary endosymbionts of aphids. Int J Syst Evol Microbiol 41:566–568
Muyzer G, Brinkhoff T, Nubel U, Santegoeds C, Schafer H, Wawer C (1998) Denaturing gradient gel electrophoresis (DGGE) in microbial ecology. In: Akkermans ADL, van Elsas JD, de Bruijn FJ (eds) Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, pp 3.4.4.1–3.4.4.27
Nardon P, Grenier AM (1989) Endosymbiosis in Coleoptera: biological, biochemical, and genetic aspects pp. In: Schwemmler W, Gassner G (eds) Insect endocytobiosis: morphology, physiology, genetics, evolution. CRC Press, Boca Raton, pp 175–216
Nguyen NH, Suh SO, Blackwell M (2007) Five novel Candida species in insect-associated yeast clades isolated from Neuroptera and other insects. Mycologia 99:842–858
Nguyen NH, Suh SO, Erbil CK, Blackwell M (2006) Metschnikowia noctiluminum sp. nov., Metschnikowia corniflorae sp. nov., and Candida chrysomelidarum sp. nov., isolated from green lacewings and beetles. Mycol Res 110:346–356
Noda H, Koizumi Y (2003) Sterol biosynthesis by symbiotes: cytochrome P450 sterol C-22 desaturase genes from yeast-like symbiotes of rice planthoppers and anobiid beetles. Insect Biochem Mol Biol 33:649–658
Noda H, Nakashima N, Koizumi M (1995) Phylogenetic position of yeast-like symbiotes of rice planthoppers based on partial 18S rDNA sequences. Insect Biochem Mol Biol 25:639–646
O’Donnell K (1992) Ribosomal DNA internal transcribed spacers are highly divergent in the phytopathogenic ascomycete Fusarium sambucinum (Gibberella pulicaris). Curr Genet 22:213–220
Oliver KM, Campos J, Moran NA, Hunter MS (2008) Population dynamics of defensive symbionts in aphids. Proc. Roy Soc L B Biol Sci 275:293–299
Oliver KM, Russell JA, Moran NA, Hunter MS (2003) Facultative bacteria in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA 100:1803–1807
Paul K, Nonoh JO, Mikulski L, Brune A (2012) “Methanoplasmatales,” Thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of methanogens. Appl Environ Microbiol 78:8245–8253
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Sasaki T, Kawamura M, Ishikawa H (1996) Nitrogen recycling in the brown planthopper, Nilaparvata lugens: involvement of yeast-like endosymbionts in uric acid metabolism. J Insect Physiol 42:125–129
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger JJ, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Sforza R, Boudon-Padieu E, Greif C (2003) New mealybug species vectoring Grapevine leafroll-associated viruses-1 and-3 (GLRaV-1 and-3). Eur J Plant Pathol 109:975–981
Shen SK, Dowd PF (1992) Detoxifying enzymes and insect symbionts. J Chem Educ 69:796–799
Singh ST, Kumar J, Thomas A, Ramamurthy VV, Rajagopal R (2013) Detection and localization of Rickettsia sp. in mealybug. Environ Entomol 42:711–716
Staubach F, Baines JF, Künzel S, Bik EM, Petrov DA (2013) Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS One 8:e70749
Suh SO, Gibson CM, Blackwell M (2004) Metschnikowia chrysoperlae sp. nov., Candida picachoensis sp. nov. and Candida pimensis sp. nov., isolated from the green lacewings Chrysoperla comanche and Chrysoperla carnea (Neuroptera: Chrysopidae). Int J Syst Evol Microbiol 54:1883–1890
Takai K, Horikoshi K (2000) Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66:5066–5072
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tang M, Lv L, Jing S, Zhu L, He G (2010) Bacterial symbionts in the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Appl Environ Microbiol 76:1740–1745
Tanne E, Ben-Dov Y, Raccah B (1989) Transmission of the corky-bark disease by the mealybug Planococcus ficus. Phytoparasitica 17:55
Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:2753–2763
Tsai CW, Chau J, Fernandez L, Bosco D, Daane KM, Almeida RPP (2008) Transmission of Grapevine leafroll-associated virus 3 by the vine mealybug (Planococcus ficus). Phytopatology 98:1093–1098
van den Heuvel JFJM, Verbeek M, van der Wilk F (1994) Endo-symbiotic bacteria associated with circulative transmission of potato leafroll virus by Myzus persicae. J Gen Virol 75:2559–2565
Vega FE, Dowd PF (2005) The role of yeasts as insect endosymbionts pp. In: Vega FE, Blackwell M (eds) Insect-fungal associations: ecology and evolution. University Press, New York, Oxford, pp 211–243
von Dohlen CD, Kohler S, Alsop ST, McManus WR (2001) Mealybug beta-proteobacterial endosymbionts contain gamma-proteobacterial symbionts. Nature 412:433–436
Walton VM (2003) Development of an integrated pest management system for vine mealybug, Planococcus ficus (Signoret), in vineyards in the Western Cape Province, South Africa. Dissertation (PhD), Stellenbosch University
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, pp 315–322
Woolfolk SW, Inglis GD (2004) Microorganisms associated with field-collected Chrysoperla rufilabris (Neuroptera: Chrysopidae) adults with emphasis on yeast symbionts. Biol Cont 29:155–168
Yaman M, Radek R (2008) Identification, distribution and occurrence of the ascomycete Metschnikowia typographi in the great spruce bark beetle, Dendroctonus micans. Folia Microbiol 53:427–432
Zada A, Dunkelblum E, Assael F, Harel M, Cojocaru M, Mendel Z (2003) Sex pheromone of the vine mealybug, Planococcus ficus in Israel: occurrence of a second component in a mass-reared population. J Chem Ecol 29:977–988
Zheng L, Crippen TL, Singh B, Tarone AM, Dowd S, Yu Z, Tomberlin JK (2013) A survey of bacterial diversity from successive life stages of black soldier fly (Diptera: Stratiomyidae) by using 16S rDNA pyrosequencing. J Med Entomol 50:647–658
Acknowledgments
This research was funded by Research Grant No. CA-9110-09 from BARD, the United States-Israel Binational Agricultural Research and Development Fund to EZF, and by an National Sciences and Engineering Research Council of Canada (NSERC) Special Research Opportunity Canada-Israel Grant (SROCI-386044-2009) to SP. SP acknowledges support from the Integrated Microbial Biodiversity Program of the Canadian Institute for Advanced Research. LIK, SD, and EZF acknowledge support from the Chief Scientist, Israel Ministry of Agriculture. LIK acknowledges support from the Lady Davis Fellowship Trust, Technion. Thanks are extended to Eduard Belausov for technical support and Prof. Oded Beja for scientific support. The comments from two anonymous reviewers greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Steve J Perlman and Einat Zchori-Fein both are co-senior authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Iasur-Kruh, L., Taha-Salaime, L., Robinson, W.E. et al. Microbial Associates of the Vine Mealybug Planococcus ficus (Hemiptera: Pseudococcidae) under Different Rearing Conditions. Microb Ecol 69, 204–214 (2015). https://doi.org/10.1007/s00248-014-0478-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0478-2