Abstract
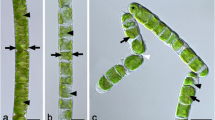
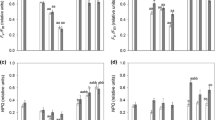
Members of the cosmopolitan green algal genus Klebsormidium (Klebsormidiales, Streptophyta) are typical components of terrestrial microbiotic communities such as biological soil crusts, which have many important ecological functions. In the present study, Klebsormidium dissectum (Gay) Ettl & Gärtner was isolated from a high alpine soil crust in the Tyrolean Alps, Austria. Physiological performance in terms of growth and photosynthesis was investigated under different controlled abiotic conditions and compared with ultrastructural changes under the treatments applied. K. dissectum showed very low light requirements as reflected in growth patterns and photosynthetic efficiency. Increasing temperatures from 5°C to 40°C led to different effects on respiratory oxygen consumption and photosynthetic oxygen evolution. While at low temperatures (5–10°C), respiration was not detectable or on a very low level, photosynthesis was relatively high, Reversely, at the highest temperature, respiration was unaffected, and photosynthesis strongly inhibited pointing to strong differences in temperature sensitivity between both physiological processes. Although photosynthetic performance of K. dissectum was strongly affected under short-term desiccation and recovered only partly after rehydration, this species was capable to survive even 3 weeks at 5% relative air humidity. K. dissectum cells have a cell width of 5.6 ± 0.3 μm and a cell length of 8.4 ± 2.0 μm. Desiccated cells showed a strongly reduced cell width (46% of control) and cell length (65% of control). In addition, in desiccated cells, fewer mitochondria were stained by DIOC6, and damaged plasma membranes were detected by FM 1–43 staining. High-pressure freeze fixation as well as chemical fixation allowed visualizing ultrastructural changes caused by desiccation. In such cells, the nucleus and chloroplast were still visibly intact, but the extremely thin cell walls (75–180 nm) were substantially deformed. The cytoplasm appeared electron dense and mitochondria were altered. Although K. dissectum can be characterized as euryoecious species, all ecophysiological and ultrastructural data indicate susceptibility to desiccation. However, the steadily occurring fragmentation of filaments into smaller units leads to improved self protection and thus may represent a life strategy to better survive longer periods of drought in exposed alpine soil crusts.










Similar content being viewed by others
References
Abe K, Mihara H, Hirano M (1998) Characteristics of growth and carotenoid accumulation of the aerial microalga Trentepohlia aurea in liquid culture. J Mar Biotechnol 6:53–58
Belnap J, Lange OL (2001) Biological soil crusts: structure. Function and Management. Springer, Berlin, Germany, p 503
Bischof K, Hanelt D, Tüg H, Karsten U, Brouwer PEM, Wiencke C (1998) Acclimation of brown algal photosynthesis to ultraviolet radiation in Arctic coastal waters (Spitsbergen, Norway). Polar Biol 20:388–395
Büdel B (2001) Biological soil crusts of European temperate and Mediterranean regions. In: Belnap J, Lange OL (eds) Biological soil crusts: structure. Function and Management, Springer, Berlin, pp 75–85
Cardon ZG, Gray DW, Lewis LA (2008) The green algal underground: evolutionary secrets of desert cells. BioSci 58:114–122
Darienko T, Gustavs L, Mudimu O, Menendez CR, Schumann R, Karsten U, Friedl T, Pröschold T (2010) Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta). Eur J Phycol 45:1–17
Davey MC (1989) The effects of freezing and desiccation on photosynthesis and survival of terrestrial Antarctic algae and cyanobacteria. Polar Biol 10:29–36
De Winder B, Matthijs HCP, Mur LR (1990) The effect of dehydration and ion stress on carbon dioxide fixation in drought-tolerant phototrophic micro-organisms. FEMS Microbiol Ecol 74:33–38
Domozych DS, Roberts R, Danyow C, Fltter R, Smith B, Providence K (2003) Plasmolysis, hechtian strand formation, and localized membrane-wall adhesions in the desmid, Closterium acerosum (Chlorophyta). J Phycol 39:1194–1206
Elbert W, Weber B, Büdel B, Andreae MO, Pöschl U (2009) Microbiotic crusts on soil, rock and plants: neglected major players in the global cycles of carbon and nitrogen? Biogeosci Discuss 6:6983–7015
Elster J, Degma P, Kovacik L, Valentova L, Sramkova K, Pereira AB (2008) Freezing and desiccation injury resistance in the filamentous green alga Klebsormidium from the Antarctic, Arctic and Slovakia. Biologia 63:839–847
Ettl H, Gärtner G (1995) Syllabus der Boden-, Luft- und Flechtenalgen. Gustav Fischer, Stuttgart, p 721
Evans RD, Johansen JR (1999) Microbiotic crusts and ecosystem processes. Crit Rev Plant Sci 18:83–225
Gärtner G (2004) ASIB—the culture collection of algae at the Botanical Institute, Innsbruck, Austria. Nova Hed 79:71–76
Gray DW, Lewis LA, Cardon ZG (2007) Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant Cell Environ 30:1240–1255
Green TGA, Nash TH III, Lange OL (2008) Physiological ecology of carbon dioxide exchange. In: Nash TH III (ed) Lichen biology. Cambridge University Press, Cambridge, pp 152–181
Gustavs L, Schumann R, Eggert A, Karsten U (2009) In vivo growth fluorometry: accuracy and limits of microalgal growth rate measurements in ecophysiological investigations. Aqua Microb Ecol 55:95–104
Häubner N, Schumann R, Karsten U (2006) Aeroterrestrial algae growing on facades—response to temperature and water stress. Microb Ecol 51:285–293
Holzinger A (2009) Desiccation tolerance in green algae: implications of physiological adaptation and structural requirements. In: Hagen KN (ed) Algae, nutrition, pollution control and energy sources. Nova, Hauppauge, New York, pp 41–56
Holzinger A, Karsten U, Lütz C, Wiencke C (2006) Ultrastructure and photosynthesis in the supralittoral green macroalga Prasiola crispa (Lightfoot) Kützing from Spitsbergen (Norway) under UV exposure. Phycologia 45:168–177
Holzinger A, Roleda M, Lütz C (2009) The vegetative arctic green alga Zygnema is insensitive to experimental UV exposure. Micron 40:831–838
Holzinger A, Tschaikner A, Remias D (2010) Cytoarchitecture of the desiccation-tolerant green alga Zygogonium ericetorum. Protoplasma 243:15–24
Holzinger, A, Lütz, C, Karsten, U (2011). Desiccation stress causes structural and ultra-structural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. J Phycol 47:591–602. doi:10.1111/j.1529-8817.2011.00980.x
Honda M, Hashimoto H (2007) Close association of centrosomes to the distal ends of the microbody during its growth, division and partitioning in the green alga Klebsormidium flaccidum. Protoplasma 231:127–135
Karsten U, Friedl T, Schumann R, Hoyer K, Lembcke S (2005) Mycosporine-like amino acids (MAAs) and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). J Phycol 41:557–566
Karsten U, Lütz C, Holzinger A (2010) Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. J Phycol 46:1187–1197
Körner C (2003) Alpine plant life—functional plant ecology of high mountain ecosystems. Springer, Berlin, p 344
Kouril R, Ilik P, Tomek P, Naus J, Poulickova A (2001) Chlorophyll fluorescence temperature curve on Klebsormidium flaccidum cultivated at different temperature regimes. J Plant Physiol 158:1131–1136
Kühl M, Jörgensen BB (1994) The light field of microbenthic communities: radiance distribution and microscale optics of sandy coastal sediments. Limnol Oceanogr 39:1368–1398
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional. Springer, Berlin, p 513
Larcher W, Wagner J (2009) High mountain bioclimate: temperatures near the ground recorded from the timber-line to the nival zone in the Central Alps. Contrib Nat Hist 12:857–874
Lewis LA (2007) Chlorophyta on land: independent lineages of green eukaryotes from arid lands. In: Seckbach J (ed) Algae and Cyanobacteria in extreme environments. Springer, Berlin
Lukesova A (2001) Soil algae in brown coal and lignite post-mining areas in central Europe (Czech Republic and Germany). Restor Ecol 9:341–350
Lüttge U, Büdel B (2010) Resurrection kinetics of photosynthesis in desiccation-tolerant terrestrial green algae (Chlorophyta) on tree bark. Plant Biol 12:437–444
Morison MO, Sheath RG (1985) Responses to desiccation stress by Klebsormidium rivulare (Ulotrichales, Chlorophyta) from a Rhode Island stream. Phycologia 24:129–145
Nagao M, Matsui K, Uemura M (2008) Klebsormidium flaccidum, a charophycean green alga, exhibits cold acclimation that is closely associated with compatible solute accumulation and ultrastructural changes. Plant Cell Environ 31:872–885
Porra RJ, Thompson WA, Kriedmann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
ap Rees T, Burrell MM, Entwistle TG, Hammond JB, Kirk D, Kruger NJ (1988) Effects of low temperature on the respiratory metabolism of carbohydrates by plants. Symp Soc Exp Biol 42:377–393
Reisigl H (1964) Zur Systematik und Ökologie alpiner Bodenalgen. Österr Bot Z 111:402–499
Remias D, Albert A, Lütz C (2010) Effects of simulated, but realistic, elevated UV irradiation on photosynthesis and pigment composition of the alpine snow alga Chlamydomonas nivalis and the Arctic soil alga Tetracystis sp. (Chlorophyceae). Photosynthetica 48:269–277
Rindi F, Guiry MD, Lopez-Bautista JM (2008) Distribution, morphology, and phylogeny of Klebsormidium (Klebsormidiales, Charophyceae) in urban environments in Europe. J Phycol 44:1529–1540
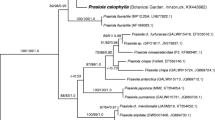
Rindi F, Mikhailyuk TI, Sluiman HJ, Friedl T, Lopez-Bautista JM (2011) Phylogenetic relationships in Interfilum and Klebsormidium (Klebsormidiophyceae, Streptophyta). Mol Phylogen Evol 58:218–231
Starr RC, Zeikus JA (1993) UTEX—the culture collection of algae at the University of Texas at Austin 1993 list of cultures. J Phycol 29:1–106
Tan CK, Lee YK, Ho KK (1993) Effect of light intensity and ammonium-N on carotenogenesis of Trentepohlia odorata and Dunaliella bardawil. J Appl Phycol 5:547–549
Tschaikner A (2008) Soil algae and soil algal crusts in the alpine regions of Tyrol (Ötztal, Austria). Ph.D. thesis, University of Innsbruck, p 58
Tschaikner A, Ingolic E, Holzinger A, Gärtner G (2007) Phycobionts of some species of Evernia and Ramalina. Herzogia 20:53–60
Türk R, Gärtner G (2001) Biological soil crusts in the subalpine, alpine, and nival areas in the Alps. In: Belnap J, Lange OL (eds) Biological soil crusts: structure. Function and Management, Springer, Berlin, pp 67–73
Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra: a mathematical model. Oecologia 17:281–291
Acknowledgements
The present investigation was undertaken during the first author's sabbatical at the University of Innsbruck in Prof. Cornelius Lütz's laboratory. We thank W. Kofler, Institute of Botany, University of Innsbruck for SEM preparations. Prof. U. Lütz-Meindl, University of Salzburg is kindly acknowledged for providing access to her Leica high-pressure freezing device. We thank Mag. A. Andosch, University of Salzburg for technical help with the freeze substitution. Dr. Thomas Pröschold, University of Vienna, undertook the ITS2 rRNA analysis. Financial support by the Deutsche Forschungsgemeinschaft (DFG) (KA899/16-1/2) is gratefully acknowledged by U.K. The study has been supported by a Research Grant from the “Universitätszentrum Obergurgl”, University of Innsbruck to A.H. The authors are grateful to Dr. Daniel Remias for technical support with the photosynthetic oxygen measurements. The authors like to thank Dr. Angelika Tschaikner and Prof. Georg Gärtner for species identification, as well as Prof. Christian Wiencke for lending the PAM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karsten, U., Holzinger, A. Light, Temperature, and Desiccation Effects on Photosynthetic Activity, and Drought-Induced Ultrastructural Changes in the Green Alga Klebsormidium dissectum (Streptophyta) from a High Alpine Soil Crust. Microb Ecol 63, 51–63 (2012). https://doi.org/10.1007/s00248-011-9924-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9924-6