Abstract
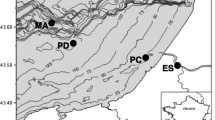
Sulfate- and sulfite-reducing prokaryotes (SSRP) communities play a key role in both sulfur and carbon cycles. In estuarine ecosystems, sulfate concentrations change with tides and could be limited in tidal freshwater reach or deep sediments. In a subtropical estuary of northern Taiwan in December 2007, we examined the compositional changes of SSRP communities. We examined three sites: from the lower estuarine brackish-water reach (site GR and mangrove vegetation site, GM) to the upper estuarine tidal freshwater reach (site HR), as well as from surface to a 50-cm depth. The partial sequence of sulfite reductase (dsrB) genes was used as a molecular marker of SSRP, linked to polymerase chain reaction and denaturing gradient gel electrophoresis (DGGE) techniques. SSRP communities of the DGGE profiles varied with sites according to one-way analyses of similarities (Global R = 0.69, P = 0.001). Using cluster analysis, the DGGE profile was found to show site-specific clusters and a distinct depth zonation (five, six, and two SSRP communities at the GM, GR, and HR sites, respectively). SSRP composition was highly correlated to the combination of salinity, reduced sulfur, and total organic carbon contents (BIO-ENV analysis, r s = 0.56). After analyzing a total of 35 dsrB sequences in the DGGE gel, six groups with 15 phylotypes were found, which were closely related to marine-freshwater gradient. Moreover, sequences neighboring sulfite-reducing prokaryotes were observed, in addition to those affiliated to sulfate-reducing prokaryotes. Four phylotypes harvested in HR resembled the genus Desulfitobacterium, a sulfite-reducing prokaryote, which failed to use sulfate as an electron acceptor and were active in freshwater and sulfate-limited habitat. The other five phylotypes in the HR reach belonged to the sulfate-reducing prokaryotes of the genera Desulfatiferula, Desulfosarcina, Desulfovibrio, and Desulfotomaculum, which appeared to tolerate low salinity and low sulfate supply. SSRP phylotypes at the mangrove-vegetated GM site (five phylotypes in two groups) were phylogenetically less diverse, when compared with those at the non-mangrove-vegetated GR site (three phylotypes in three groups) and the tidally influenced freshwater HR site (nine phylotypes in five groups). Phylotypes found at GR and GM were all affiliated to marine sulfate-reducing prokaryote strains of the genera Desulfofaba, Desulfobotulus, Desulfatiferula, Desulfosarcina, and Desulfotomaculum. Notably, a phylotype recorded in the surface sediment at GR resembled the genus Desulfobulbus, which was recorded from freshwater environment consisting of the freshwater input at GR during ebb tides.





Similar content being viewed by others
References
Abildgaard L, Ramsing NB, Finster K (2004) Characterization of the marine propionate-degrading, sulfate-reducing bacterium Desulfofaba fastidiosa sp. nov. and reclassification of Desulfomusa hansenii as Desulfofaba hansenii comb. nov. Int J Syst Evol Microbiol 54:393–399
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Baena S, Fardeau ML, Labat M, Ollivier B, Garcia JL, Patel BK (1998) Desulfovibrio aminophilus sp. nov., a novel amino acid degrading and sulfate reducing bacterium from an anaerobic dairy wastewater lagoon. Syst Appl Microbiol 21(4):498–504
Bahr M, Crump BC, Klepac-Ceraj V, Teske A, Sogin ML, Hobbie JE (2005) Molecular characterization of sulfate-reducing bacteria in a New England salt marsh. Environ Microbiol 7:1175–1185
Barton LL, Fauque GD (2009) Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv Appl Microbiol 68:41–98
Bryant MP, Campbell LL, Reddy CA, Crabill MR (1977) Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol 33:1162–1169
Canfield DE, Thamdrup B, Kristensen E (2005) Aquatic geomicrobiology. Adv Mar Biol 48:1–600
Capone DG, Kiene RP (1988) Comparison of microbial dynamics in marine and freshwater sediments: contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33:725–749
Castro H, Reddy KR, Ogram A (2002) Composition and function of sulfate-reducing prokaryotes in eutrophic and pristine areas of the Florida Everglades. Appl Environ Microbiol 68:6129–6137
Chartrain M, Zeikus JG (1986) Microbial ecophysiology of whey biomethanation: characterization of bacterial trophic populations and prevalent species in continuous culture. Appl Environ Microbiol 51:188–196
Chen CP, Wu JT, Lin S, Lin, HJ, Hsieh HL, Liu WC, Chen CC, Li LA (2001) A suitable plan for the sediment removal to reduce the oxygen exhaustion in the main stream Tanshui River and its tributary Keelung River. Taiwan Environmental Protection Administration, Taipei, Taiwan. EPA-90-G107-02-104 (in Chinese)
Christiansen N, Ahring BK (1996) Desulfitobacterium hafniense sp. nov. an anaerobic, reductively dechlorinating bacterium. Int J Syst Bacteriol 46:442–448
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
Clarke KR, Gorley RN (2001) ‘Primer V5: User Manual/Tutorial. Plymouth, Plymouth Marine Laboratory
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14(3):454–458
Cottrell MT, Cary SC (1999) Diversity of dissimilatory sulfite reductases genes of bacteria associated with deep-sea hydrothermal vent polychaete annelid Alvinella pompejana. Appl Environ Microbiol 65:1127–1132
Cravo-Laureau C, Labat C, Joulian C, Matheron R, Hirschler-Réa A (2007) Desulfatiferula olefinivorans gen. nov., sp. nov., a long-chain n-alkene-degrading, sulfate-reducing bacterium. Int J Syst Evol Microbiol 57:2699–2702
Devereux R, Delaney M, Widdle F, Stahl DA (1989) Natural relationships among sulfate-reducing Eubacteria. J Bacteriol 171:6689–6695
Dilling W, Cypionka H (1990) Aerobia respiration in sulfate-reducing bacteria. FEMS Microbiol Lett 71:123–127
Dhillon A, Teske A, Dillon J, Stahl DA, Sogin ML (2003) Molecular characterization of sulfate-reducing bacteria in the Guaymas basin. Appl Environ Microbiol 69:2765–2772
Fan LF, Shieh WY, Wu WF, Chen C-P (2006) Distribution of nitrogenous nutrients and denitrifier strains in estuarine sediment profiles of the Tanshui River, northern Taiwan. Estuar Coast Shelf Sci 69(3–4):543–553
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376
Fitch WM (1971) Toward defining the course of evolution: minimum change for specified tree topology. Syst Zool 20:406–416
Geets J, Borrernans B, Diels L, Springael D, Vangronsveld J, van der Lelie D, Vanbroekhoven K (2006) DsrB gene-based DGGE for community and diversity surveys of sulfate-reducing bacteria. J Microbiol Meth 66:194–205
Hipp WM, Pott A, Thum-Schmitz N, Faath I, Dahl C, Trüper HG (1997) Towards the phylogeny of APS reductases and sirohaem sulfite reductases in sulfate-reducing and sulfur-oxidizing prokaryotes. Microbiology 143:2891–2902
Holmer M, Storkholm P (2001) Sulphate reduction and sulphur cycling in lake sediments: a review. Freshwater Biol 46:431–451
Hsieh HL (1995) Spatial and temporal patterns of polychaete communities in a subtropical mangrove swamp—influences of sediment and microhabitat. Mar Ecol Prog Ser 127:157–167
Hsieh HL, Chen CP, Chen YG, Yang HH (2002) Diversity of benthic organic matter flows through polychaetes and crabs in a mangrove estuary: 13 C and 34S signals. Mar Ecol Prog Ser 227:145–155
Hsieh H-L, Fan L-F, Chen C-P, Wu J-T, Liu W-C (2010) Effects of semidiurnal tidal circulation on the distribution of holo- and meroplankton in a subtropical estuary. J plankton Res 32(6):829–841
Hsieh YP, Yang CH (1997) Pyrite accumulation and sulfate depletion affected by root distribution in a Juncus (Needle Rush) Salt Marsh. Estuaries 20(3):640–645
Hsu M-S, Fu JC, Lin S-H (2001) The influence of garbage dump on flood in Tanshui River. J Taiwan Conservancy 49(4):1–13 (in Chinese)
Huang SC, Shih SS, Ho YS, Chen CP, Hsieh HL (2011) Restoration of shorebird-roosting mudflats by partial removal of estuarine mangroves in northern Taiwan. Restor Ecol (in press)
Ito T, Okabe S, Satoh H, Watanabe Y (2002) Successional development of sulfate-reducing bacterial populations and their activities in a wastewater biofilm growing under microaerophilic conditions. Appl Environ Microbiol 68:1392–1402
Jiang L, Zheng Y, Peng X, Zhou H, Zhang C, Xiao X, Wang D (2009) Vertical distribution and diversity of sulfate-reducing prokaryotes in the Pearl River estuarine sediments, Southern China. FEMS Microbiol Ecol 70:249–262
Jonkers HM, Koh IO, Behrend P, Muyzer G, de Beer D (2005) Aerobic organic carbon mineralization by sulfate-reducing bacteria in the oxygen-saturated photic zone of a hypersaline microbial mat. Microb Ecol 49:291–300
Karkhoff-Schweizer RR, Huber DPW, Voordouw G (1995) Conservation of the genes for dissimilatory sulfite reductase from Desulfovibrio vulgaris and Archaeoglobus fulgidus allows their detection by PCR. Appl Environ Microbiol 61:290–296
Klein M, Friedrich M, Roger AJ, Hugenholtz P, Fishbain S, Abicht H, Blackall LL, Stahl DA, Wagner M (2001) Multiple lateral transfers of dissimilatory sulfite reductase genes between major lineages of sulfate-reducing prokaryotes. J Bacteriol 183:6028–6035
Kuever J, Rainey F, Widdel F (2005) Desulfovibrio Kluyver and van Niel 1936, 397 AL. In: Brenner DJ, Krieg NR, Garrity GM, Staley JT, Boone DR, Vos PD, Goodfellow M, Rainey FA, Schleifer K-H (eds) Bergey’s Manual® of Systematic Bacteriology, Vol. 2, The Proteobacteria, Part C, The Alpha-, Beta-, Delta-, and Epsilonproteobacteria. Springer, New York, USA, pp 926–938
Kuever J, Rainey F, Widdel F (2005) Desulfosarcina Widdel 1981, 382VP. In: Brenner DJ, Krieg NR, Garrity GM, Staley JT, Boone DR, Vos PD, Goodfellow M, Rainey FA, Schleifer K-H (eds) Bergey’s Manual® of Systematic Bacteriology, Vol. 2, The Proteobacteria, Part C, The Alpha-, Beta-, Delta-, and Epsilonproteobacteria. Springer, New York, USA, pp 981–984
Kuever J, Rainey F, Widdel F (2005) Desulfobulbus Widdel 1981, 382 VP (Effective publication: Widdel 1980, 374). In: Brenner DJ, Krieg NR, Garrity GM, Staley JT, Boone DR, Vos PD, Goodfellow M, Rainey FA, Schleifer K-H (eds) Bergey’s Manual® of Systematic Bacteriology, Vol. 2, The Proteobacteria, Part C, The Alpha-, Beta-, Delta-, and Epsilonproteobacteria. Springer, New York, USA, pp 988–992
Laanbroek HJ (2010) Methane emission from natural wetlands: interplay between emergent macrophytes and soil microbial processes. A mini-review. Ann Bot 105:141–153
Leloup J, Quillet L, Berthe T, Petit F (2006) Diversity of the dsrAB (dissimilatory sulfite reductase) gene sequences retrieved from two contrasting mudflats of the Seine estuary, France. FEMS Microbiol Ecol 55:230–238
Leloup J, Loy A, Knab NJ, Borowski C, Wagner M, Jørgensen BB (2007) Diversity and abundance of sulfate-reducing microorganisms in the sulfate and methane zones of a marine sediment, Black Sea. Environ Microbiol 9:131–142
Liu WC, Hsu MH, Kuo AY (2002) Modeling of hydrodynamics and cohesive sediment transport in Tanshui River estuarine system, Taiwan. Mar Pollu Bull 44:1076–1088
Loy A, Kusel K, Lehner A, Drake H, Wagner M (2004) Microarray and functional gene analyses of sulfate-reducing prokaryotes in low-sulfate, acidic fens reveal cooccurrence of recognized genera and novel lineages. Appl Environ Microbiol 70:6998–7009
Meyer B, Kuever J (2007) Molecular analysis of the distribution and phylogeny of dissimilatory adenosine-59-phosphosulfate reductase-encoding genes (aprBA) among sulfuroxidizing prokaryotes. Microbiology 153:3478–3498
Meysman FJR, Middelburg JJ, Heip CHR (2006) Bioturbation: a fresh look at Darwin’s last idea. Trends Ecol Evol 21(12):688–695
Miletto M, Loy A, Antheunisse AM, Loeb R, Bodelier PLE, Laanbroek HJ (2008) Biogeography of sulfate-reducing prokaryotes in river floodplains. FEMS Microbiol Ecol 64:395–406
Minz D, Flax JL, Green SJ, Muyzer G, Cohen Y, Wagner M, Rittmann BE, Stahl DA (1999) Diversity of sulfate-reducing bacteria in oxic and anoxic regions of a microbial mat characterized by comparative analysis of dissimilatory sulfite reductase genes. Appl Environ Microbiol 65:4666–4671
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes encoding for 16S rRNA. Appl Environ Microbiol 59:695–700
Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nature Rev Microbiol 6:441–454
Nercessian O, Bienvenu N, Moreira D, Prieur D, Jeanthon C (2005) Diversity of functional genes of methanogens, methanotrophs and sulfate-reducers in deep-sea hydrothermal environments. Environ Microbiol 7:118–132
Nocker A, Lepo JE, Martin LL, Snyder RA (2007) Response of estuarine biofilm microbial community development to changes in dissolved oxygen and nutrient concentrations. Microb Ecol 54:532–542
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol Rev Ann Rev 16:229–311
Rabus R, Hansen T, Widdel F (2006) Dissimilatory sulfate- and sulfur-reducing prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The Prokaryotes, Vol. 2. Springer, New York, USA, pp 659–768
Rosenberg R, Nilsson HC, Diaz RJ (2001) Response of benthic fauna and changing sediment redox profiles over a hypoxic gradient. Estuar Coast Shelf Sci 53:343–350
Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mo Biol Evol 4:406–425
Schwermer CU, Ferdelman TG, Stief P, Gieseke A, Rezakhani N, van Rijn J, de Beer D, Schramm A (2010) Effect of nitrate on sulfur transformations in sulfidogenic sludge of a marine aquaculture biofilter. FEMS Microbiol Ecol 72:476–484
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, and Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution (in press)
Tasaki M, Kamagata Y, Nakamura K, Mikami E (1991) Isolation and characterization of thermophilic benzonation of the thermophilic benzoate-degrading, sulfate-reducing bacterium, Desulfotomaculum thermobenzoicum sp. nov. Arch Microbiol 155:348–352
Thompson DB, Ravussin E, Bennett PH, Bogardus C (1997) Structure and sequence variation at the human leptin receptor gene in lean and obese Pima Indians. Hum Mol Genet 6(5):675–679
Utkin I, Woese C, Wiegel J (1994) Isolation and characterization of Desulfitobucterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Sys Bacteriol 44:612–619
Wagner M, Roger AJ, Flax JL, Brusseau GA, Stahl DA (1998) Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol 180:2975–2982
Wen LS, Jiann KT, Liu KK (2008) Seasonal variation and flux of dissolved nutrients in the Danshuei Estuary, Taiwan: a hypoxic subtropical mountain river. Estuar Coast Shelf Sci 78:694–704
Wu X-J, Pan J-L, Liu X-L, Li D-T, Yang H (2009) Sulfate-reducing bacteria in leachate-polluted aquifers along the shore of the East China Sea. Can J Microbiol 55:1–11
Yamashita T, Yamamoto-Ikemoto R, Sakurai E, Aikawa K, Kaneko E (2010) Treatment of municipal wastewater using an anaerobic–anoxic–oxic biological filter reactor packed with carbon fibers and aerated with microbubbles. Sustain Environ Res 20(4):205–211
Zhang W, Song L-S, J-S KI, Lau C-K, Li X-D, Qian P-Y (2008) Microbial diversity in polluted harbor sediments II: Sulfate-reducing bacterial community assessment using terminal restriction fragment length polymorphism and clone library of dsrAB gene. Estuar Coast Shelf Sci 76:682–691
Acknowledgments
We sincerely thank Mr. Timothy M. Davidson for reading the manuscript. The authors are very grateful to the anonymous reviewers for their constructive comments. This study was funded through Thematic Research Program by Academia Sinica, Taiwan. This study complies with the current laws of Taiwan in which it was performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sen-Lin Tang and Hwey-Lian Hsieh contributed equally.
Rights and permissions
About this article
Cite this article
Fan, LF., Tang, SL., Chen, CP. et al. Diversity and Composition of Sulfate- and Sulfite-Reducing Prokaryotes as Affected by Marine-Freshwater Gradient and Sulfate Availability. Microb Ecol 63, 224–237 (2012). https://doi.org/10.1007/s00248-011-9912-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9912-x