Abstract
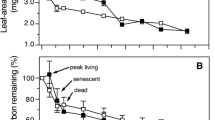
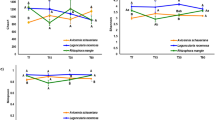
Although fungi are known to colonize and decompose plant tissues in various environments, there is scanty information on fungal communities on wetland plants, their relation to microhabitat conditions, and their link to plant litter decomposition. We examined fungal diversity and succession on Phragmites australis leaves both attached to standing shoots and decaying in the litter layer of a brackish tidal marsh. Additionally, we followed changes in fungal biomass (ergosterol), leaf nitrogen dynamics, and litter mass loss on the sediment surface of the marsh. Thirty-five fungal taxa were recorded by direct observation of sporulation structures. Detrended correspondence analysis and cluster analysis revealed distinct communities of fungi sporulating in the three microhabitats examined (middle canopy, top canopy, and litter layer), and indicator species analysis identified a total of seven taxa characteristic of the identified subcommunities. High fungal biomass developed in decaying leaf blades attached to standing shoots, with a maximum ergosterol concentration of 548 ± 83 μg g–1 ash-free dry mass (AFDM; mean ± SD). When dead leaves were incorporated in the litter layer on the marsh surface, fungi experienced a sharp decline in biomass (to 191 ± 60 μg ergosterol g–1 AFDM) and in the number of sporulation structures. Following a lag phase, species not previously detected began to sporulate. Leaves placed in litter bags on the sediment surface lost 50% of their initial AFDM within 7 months (k = −0.0035 day–1) and only 21% of the original AFDM was left after 11 months. Fungal biomass accounted for up to 34 ± 7% of the total N in dead leaf blades on standing shoots, but to only 10 ± 4% in the litter layer. These data suggest that fungi are instrumental in N retention and leaf mass loss during leaf senescence and early aerial decay. However, during decomposition on the marsh surface, the importance of living fungal mass appears to diminish, particularly in N retention, although a significant fraction of total detrital N may remain associated with dead hyphae.





Similar content being viewed by others
References
Apinis, AE, Chesters, CGC, Taligoola, HK (1972) Colonisation of Phragmites communis leaves by fungi. Nova Hedwig 23: 113–124
Barnett, HL, Hunter, BB (1998) Illustrated Genera of Imperfect Fungi, 4th edn. APS Press, St. Paul, MN
Blair, JM (1988) Nitrogen, sulfur and phosphorus dynamics in decomposing deciduous leaf litter in the southern Appalachians. Soil Biol Biochem 20: 693–701
Biondini, ME, Mielke, PW Jr, Berry, KJ (1988) Data-dependent permutation techniques for the analysis of ecological data. Vegetatio 75: 161–168
Boulton, AJ, Boon, PI (1991) A review of methodology used to measure leaf litter decomposition in lotic environments—time to turn over an old leaf. Aust J Mar Freshw Res 42: 1–43
Buesing, N, Gessner, MO (2006) Benthic bacterial and fungal productivity and carbon turnover in a freshwater marsh. Appl Environ Microbiol 72: 596–605
Buchan, A, Newell, SY, Moreta, JIL, Moran, MA (2002) Analysis of internal transcribed spacer (ITS) regions of rRNA genes in fungal communities in a southeastern U.S. Salt Marsh. Microb Ecol 43: 329–340
Casas, JJ, Gessner, MO (1999) Leaf litter breakdown in a Mediterranean stream characterised by travertine precipitation. Freshw Biol 41: 781–793
Cooke, RC, Rayner, ADM (1984) Ecology of Saprotrophic Fungi. Longman, London, UK
Cowie, NR, Sutherland, WJ, Ditlhogo, KM, James, R (1992) The effects of conservation management of reed beds. II. The flora and litter disappearance. J Appl Ecol 29: 277–284
De Mesel, I, Derycke, S, Swings, J, Vincx, M, Moens, T (2003) Influence of bacterivorous nematodes on the decomposition of cordgrass. J Exp Mar Biol Ecol 296: 227–242
Dennis, RWG (1981) British Ascomycetes. J. Cramer, London, UK
Dufrêne, M, Legendre, P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67: 345–366
Ellis, MB (1971) Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, UK
Ellis, MB (1976) More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, UK
Ellis, MB, Ellis, JP (1997) Microfungi on Land Plants. An identification handbook, new enlarged edition. Richmond Publishing, London, UK
Fell, JW, Hunter, IL (1979) Fungi associated with the decomposition of Juncus roemerianus in South Florida. Mycologia 71: 322–342
Findlay, SEG, Dye, S, Kuehn, KA (2002) Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22: 616–625
Frankland, JC (1998) Fungal succession—unraveling the unpredictable. Mycol Res 102: 1–15
Gessner, MO (2000) Breakdown and nutrient dynamics of submerged Phragmites shoots in the littoral zone of a temperate hardwater lake. Aquat Bot 66: 9–20
Gessner, MO (2001) Mass loss, fungal colonisation and nutrient dynamics of Phragmites australis leaves during senescence and early decay. Aquat Bot 69: 325–339
Gessner, MO, Newell, SY (2002) Biomass, growth rate, and production of filamentous fungi in plant litter. In: Hurst, CJ, Crawford, RL, Knudsen, GR, McInerney, MJ, Stetzenbach, LD (Eds.) Manual of Environmental Microbiology, 2nd edn. ASM Press, Washington DC, USA, pp 390–408
Gessner, MO, Schmitt, AL (1996) Use of solid-phase extraction to determine ergosterol concentrations in plant tissue colonized by fungi. Appl Environ Microbiol 62: 415–419
Gessner, MO, Van Ryckegem, G (2003) Water fungi as decomposers in freshwater ecosystems. In: Bitton, G (Ed.) Encyclopedia of Environmental Microbiology. Wiley, New York (online edition: DOI: 10.1002/0471263397.env314)
Gessner, MO, Thomas, M, Jean-Louis, AM, Chauvet, E (1993) Stable successional patterns of aquatic hyphomycetes on leaves decaying in a summer cool stream. Mycol Res 97: 63–172
Gessner, RV (1977) Seasonal occurrence and distribution of fungi associated with Spartina alterniflora from a Rhode Island estuary. Mycologia 69: 477–491
Granéli, W (1990) Standing crop and mineral content of reed, Phragmites australis (Cav.) Trin. ex Steud., in Sweden—management of reed stands to maximize harvestable biomass. Folia Geobot Phytotaxon 25: 291–302
Hietz, P (1992) Decomposition and nutrient dynamics of reed (Phragmites australis (Cav.) Trin. ex Steud.) litter in Lake Neusiedl, Austria. Aquat Bot 43: 211–230
Hudson, HJ, Webster, J (1958) Succession of fungi on decaying stems of Argopyron repens. Trans Br Mycol Soc 41: 165–177
Hunt, HW, Reuss, DE, Elliott, ET (1999) Correcting estimates of root chemical composition for soil contamination. Ecology 80: 702–707
Kirk, PM, David, JC, Stalpers, JA (2001) Ainsworth and Bisby's Dictionary of the Fungi (Including the Lichens), 9th edn. CAB International
Kohlmeyer, J, Volkmann-Kohlmeyer, B (2001) The biodiversity of fungi on Juncus roemerianus. Mycol Res 105: 1411–1412
Komínková, D, Kuehn, KA, Büsing, N, Steiner, D, Gessner, MO (2000) Microbial biomass, growth, and respiration associated with submerged litter of Phragmites australis decomposing in a littoral reed stand of a large lake. Aquat Microb Ecol 22: 271–282
Krebs, CJ (1998) Ecological Methodology, 2nd edn. Addison Wesley Longman, Menlo Park, CA
Kuehn, KA, Suberkropp, K (1998) Decomposition of standing litter of the freshwater emergent macrophyte Juncus effusus. Freshw Biol 40: 717–727
Kuehn, KA, Suberkropp, K (1998) Diel fluctuations in rates of CO2 evolution from standing dead leaf litter of the emergent macrophyte Juncus effusus. Aquat Microb Ecol 14: 171–182
Kuehn, KA, Churchill, PF, Suberkropp, K (1998) Osmoregulatory strategies of fungal populations inhabiting standing dead litter of the emergent macrophyte Juncus effusus. Appl Environ Microbiol 64: 607–612
Kuehn, KA, Lemke, MJ, Suberkropp, K, Wetzel, RG (2000) Microbial biomass and production associated with decaying leaf litter of the emergent macrophyte Juncus effesus. Limnol Oceanogr 45: 862–870
Kuehn, KA, Steiner, D, Gessner, MO (2004) Diel mineralization patterns of standing-dead plant litter: implications for CO2 flux from wetlands. Ecology 85: 2504–2518
Kufel, I, Kufel, L (1988) In situ decomposition of Phragmites australis Trin. ex Steudel and Typha angustifolia L. Ekol Pol 36: 459–470
Larsen, VJ, Schierup, HH (1981) Macrophyte cycling of zinc, copper, lead and cadmium in the littoral zone of a polluted and a non-polluted lake. II. Seasonal changes in heavy metal content of above-ground biomass and decomposing leaves of Phragmites australis (Cav.) Trin. Aquat Bot 11: 211–230
Lee, SY (1990) Net aerial primary productivity, litter production and decomposition of the reed Phragmites communis in a nature reserve in Hong Kong: management implications. Mar Ecol Prog Ser 66: 161–173
McCune, B, Grace, JB (2002) Analysis of Ecological Communities. MjM Software Design, Gleneden Beach, OR, USA
McCune, B, Mefford, MJ (1999) PC-ORD for Windows. Multivariate Analysis of Ecological Data, Version 4.0. MjM Software, Gleneden Beach, OR, USA
Mille-Lindblom, C, von Wachenfeldt, E, Tranvik, LJ (2004) Ergosterol as a measure of living fungal biomass: persistence in environmental samples after fungal death. J Microbiol Methods 59: 253–262
Mitsch, WJ, Gosselink, JG (2000) Wetlands (3rd edn) Van Nostrand Reinhold, New York, USA
Newell, SY (2001a) Spore-expulsion rates and extents of blade occupation by Ascomycetes of the smooth-cordgrass standing-decay system. Bot Mar 44: 277–285
Newell, SY (2001b) Multiyear patterns of fungal biomass dynamics and productivity within naturally decaying smooth cordgrass shoots. Limnol Oceanogr 46: 573–583
Newell, SY (2003) Fungal content and activities in standing-decaying leaf blades of plants of the Georgia Coastal Ecosystems research area. Aquat Microb Ecol 32: 95–103
Newell, SY, Arsuffi, TL, Palm, LA (1996) Misting and nitrogen fertilization of shoots of a saltmarsh grass: effects upon fungal decay of leaf blades. Oecologia 108: 495–502
Newell, SY, Arsuffi, TL, Palm, LA (1998) Seasonal and vertical demography of dead portions of shoots of smooth cordgrass in a south-temperate saltmarsh. Aquat Bot 60: 325–335
Newell, SY, Fallon, RD, Miller, JD (1989) Decomposition and microbial dynamics for standing, naturally positioned leaves of the salt-marsh grass Spartina alterniflora. Mar Biol 101: 471–481
Newell, SY, Fallon, RD, Rodriguez, RMC, Groene, LC (1985) Influence of rain, tidal wetting and relative humidity on release of carbon dioxide by standing-dead salt-marsh plants. Oecologia 68: 73–79
Newell, SY, Moran, MA, Wicks, R, Hodson, RE (1995) Productivities of microbial decomposers during early stages of decomposition of leaves of a freshwater sedge. Freshw Biol 34: 135–148
Newell, SY, Porter, D (2000) Microbial secondary production from saltmarsh-grass shoots and its known and potential fates. In: Weinstein, MP, Kreeger, DA (Eds.) Concepts and Controversion in Tidal Marsh Ecology. Kluwer Academic Publishers, Dordrecht, pp 159–186
Petrini, O, Sieber, TN, Toti, L, Vivet, O (1992) Ecology, metabolite production, and substrate utilisation in endophytic fungi. Nat Toxins 1: 185–196
Pieczyńska, E (1972) Ecology of the eulittoral zone of lakes. Ekol Pol 20: 637–732
Polunin, NVC (1984) The decomposition of emergent macrophytes in fresh water. Adv Ecol Res 14: 115–166
Rice, WR (1990) A consensus combined P-value test and the family-wide significance of component tests. Biometrics 46: 303–308
Rodewald-Rudescu, L (1974) Das Schilfrohr. Die Binnengewässer, Band XXVII: 1–302
Samuels, AL, Glass, ADM, Ehret, DL, Menzies, JG (1991) Distribution of silicon in cucumber leaves during infection by powdery mildew fungus (Sphaerotheca fuliginea). Can J Bot 69: 140–149
Soetaert, K, Hoffmann, M, Meire, P, Starink, M, van Oevelen, D, Van Regenmortel, S, Cox, T (2004) Modeling growth and carbon allocation in two reed beds (Phragmites australis) in the Scheldt estuary. Aquat Bot 79: 211–234
Suberkropp, K (1991) Relationships between growth and sporulation of aquatic hyphomycetes on decomposing leaf litter. Mycol Res 95: 843–850
Sutton, BC (1980) The Coelomycetes. Commonwealth Mycological Institute, CABI publishing, Oxon
Tanaka, Y (1991) Microbial decomposition of reed (Phragmites communis) leaves in a saline lake. Hydrobiologia 220: 119–129
Tanaka, Y (1993) Aerobic cellulolytic bacterial-flora associated with decomposing Phragmites leaf-litter in a seawater lake. Hydrobiologia 263: 145–154
Tscharntke, T (1992) Fragmentation of Phragmites habitats, minimum viable population size, habitat suitability, and local extinction of moths, midges, flies, aphids and birds. Conserv Biol 6: 530–536
Van Damme, S, Ysebaert, T, Meire, P, Van den Bergh, E (1999) Habitatstructuren, waterkwaliteit en leefgemeenschappen in het Schelde-estuarium. Rapport IN 99/24 (in Dutch), Instituut voor Natuurbehoud, Brussels, Belgium
Van Ryckegem, G, Verbeken, A (2005a) Fungal diversity and community structure on Phragmites australis (Poaceae) along a salinity gradient in the Scheldt estuary (Belgium). Nova Hedwig 80: 173–197
Van Ryckegem, G, Verbeken, A (2005b) Fungal ecology and succession on Phragmites australis in a brackish tidal marsh. I. Leaf sheaths. Fungal Divers 19: 157–187
Van Ryckegem, G, Verbeken, A (2005c) Fungal ecology and succession on Phragmites australis in a brackish tidal marsh. II. Stems. Fungal Divers 20: 209–233
Webster, J (1956) Succession of fungi on decaying cocksfoot culms. I. J Ecol 44: 517–544
Willmer, C, Fricker, M (1996) Stomata, 2nd edn. Chapman & Hall, London, UK
Windham, L (2001) Comparison of biomass production and decomposition between Phragmites australis (common reed) and Spartina patens (salt hay grass) in brackish tidal marshes of New Jersey, USA. Wetlands 21: 179–188
Wirsel, SGR, Leibinger, W, Ernst, M, Mendgen, K (2001) Genetic diversity of fungi closely associated with common reed. New Phytol 149: 589–598
Acknowledgments
We would like to thank R. Heynderickx and A. Van Heuverswijn for helping to make litter bags, A. Van Kenhove for support with nitrogen analyses, and Dr. G. Vandriessche for technical assistance and help with analyzing HPLC chromatograms. Prof. Dr. J. Van Beeumen kindly provided the HPLC system. We appreciate the critical comments of four anonymous reviewers, which greatly improved the manuscript. This research was funded by the Institute for the Promotion of Innovation by Science and Technology in Flanders, Belgium, through a grant to G.V.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Ryckegem, G., Gessner, M.O. & Verbeken, A. Fungi on Leaf Blades of Phragmites australis in a Brackish Tidal Marsh: Diversity, Succession, and Leaf Decomposition. Microb Ecol 53, 600–611 (2007). https://doi.org/10.1007/s00248-006-9132-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9132-y