Abstract
In this study, we aimed to evaluate the effect of HPL on different parameters by different centers and urologists. While doing this, we evaluated different parameters by comparing HPL(High Power laser) and LPL(Low-power laser). This is an observational, retrospective, comparative, multicentric study of prospectively organised database. A total of 217 patients who underwent RIRS for kidney stones smaller than 2 cm in three different centers were included in the study. The patients were divided into two groups; LPL used (Group1, n:121 patients) and HPL used (Group2, n:96). Propensity score matching was done in the data analysis part. After matching, a total of 192 patients, 96 patients in both groups, were evaluated. There was no difference between the groups regarding age, gender, stone side, and stone location. The stone-free rate on the first day was 80.3% in Group 1, it was 78.1% in Group 2 (p = 0.9). In the third month, it was 90.7% in Group 1 and 87.5% in Group 2 (p:0.7).Hospitalization duration was significantly higher in Group 1. (2.35 ± 2.27 days vs. 1.42 ± 1.10 days; p < 0.001).The operation duration was 88.70 ± 29.72 min in Group1 and 66.17 ± 41.02 min in Group2 (p < 0.001). The fluoroscopy time (FT) was 90.73 ± 4.79 s in Group 1 and 50.78 ± 5.64 s in Group 2 (p < 0.001). Complications according to Clavien Classification, were similar between the groups(p > 0.05). According to our study similar SFR and complication rates were found with HPL and LPL. In addition, patients who used HPL had lower operation time, hospital stay, and fluoroscopy time than the LPL group. Although high-power lasers are expensive in terms of cost, they affect many parameters and strengthen the hand of urologists thanks to the wide energy and frequency range they offer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous nephrolithotomy (PNL), retrograde intrarenal surgery (RIRS), and extracorporeal shock wave lithotripsy (ESWL) are considered optimal options for kidney stone treatment [1]. For kidney stones smaller than 2 cm, flexible ureteroscopy (FURS) with or without shock wave lithoripsy (SWL) remain recommended options by the European Association of Urology (EAU) and the American Association of Urology (AUA) guidelines [2].
The holmium:yttrium–aluminum-garnet (Ho: YAG) laser has been considered the gold standard for laser lithotripsy in the last two decades. Today, RIRS has mainly been used with Ho: YAG laser lithotripsy. [3] Besides being used successfully in lithotripsy, prostate enucleation or ablation, endopyelotomy, tumor ablation, and bladder neck incision are the areas where Ho: YAG is used effectively in urology practice.
Low-power Ho: YAG devices (LPL); lasers with a maximum power of up to 30–35 Watts with a maximum laser pulse frequency are considered. High-power Ho: YAG devices (HPL) identify lasers with power over 35 Watts [3]. HPL features higher pulse energy and higher pulse frequency. It also has the feature of obtaining faster lithotripsy and smaller stone fragments [4]. Although its use has become widespread recently, studies on its efficacy and safety are still limited and ongoing. Our current knowledge is promising that HPL offers more effective treatment in lithotripsy with a shorter operative time.
The literature suggests that HPL may be a better option than LPL; In this study, we aimed to evaluate the effect of HPL on different parameters by evaluating multiple parameters as well as evaluating the use of HPL by different centers and urologists.
Methods
The local ethics committee approved the study (Necmettin Erbakan University ethics committee 167/2023).
Type of study
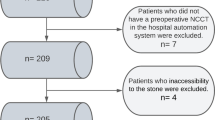
This is an observational, retrospective, comparative, multicentric study of prospectively organised database. A total of 217 patients who underwent RIRS for kidney stones smaller than 2 cm between 01 June 2021 and 1 September 2022 in 3 different centers were included in the study. The patients were divided into two groups; LPL used (Group1, n:121 patients) and HPL used (Group2, n:96). Propensity score matching was done in the data analysis part. After matching, a total of 192 patients, 96 patients in both groups, were evaluated (Fig. 1).
An informed consent form was obtained from all patients before the study. Stone characteristics, duration of surgery, Ho: YAG laser energy settings, postoperative complications, and stone-free status in preoperative non-contrast computed tomography (CT) imaging were recorded.
Inclusion exclusion criteria
Patients between the ages of 18–70 years, with kidney stones smaller than 2 cm and who underwent RIRS for kidney stones were included in the study. Patients younger than 18 years of age and older than 70 years of age, with anatomical anomalies, who had undergone ESWL before, who did not have a preoperative CT scan, whose urine culture was positive, and who did not come for follow-up at the fourth postoperative week were excluded from the study.
Devices and settings
Flex-Xc, Flex-X2s (Karl Storz, Germany) and HU-32 (Huge-med, China) flexible ureterorenoscope were used at the first Center; Flex-X2S (Karl Storz, Germany) and LithoVue (Boston Scientific, USA) in the second center; Flex-Xc, Flex-X2s (Karl Storz, Germany), Wiscope (OTU Medical) and LithoVue(Boston Scientific, USA)in the third center.
For LPL: 272 μ fiber with lasers, Dornier 30 W (Germany) in the first and second center; and 200 μ fiber with Lumenis VersaPuls P20H (Yokneam, Israel) was used in the third center. 272 μ fiber was used with HPL in the first and second center with Jenna Surgical 150 W (Germany); and 200 μ fiber with Quanta Litho Cyber Ho 150 W in the third center (Samarate, Italy).
Surgical tecnique and process
All surgical procedures were performed by experienced endourologists. The procedure was performed in the lithotomy position under general anesthesia. A safety guide wire was placed in the kidney under fluoroscopic guidance. All patients underwent ureterorenoscopy with a rigid ureterorenoscope. A second guidewire was placed during ureterorenoscopy.
In patients who used UAS, UAS was advanced over the second guide wire. In patients who did not use UAS, flexible URS was advanced directly to the kidney over the second guidewire. After reaching the stone, the stone was fragmented with the Holmium:YAG laser.
UAS was used according to the surgeon’s preference. The procedure was performed without sheath in patients whose UAS could not be placed. If the procedure could not be performed without UAS, a DJ stent was placed and tried again 1 month later. The procedure was performed after treatment in patients with a positive preoperative urine culture.
According to each departmental regulation, the Double J stent was removed 2–4 weeks after surgery. SFR was defined as the absence of fragments of any size in X-ray and USG performed at four weeks postoperatively.
Outcome measure
The primary outcome measure was stone-free rate determined by standard care imaging at three months. Intraoperative and postoperative complications, operative time, hospitalization time, and postoperative analgesia were secondary outcome measures. Amount of the energy Joule and frequency used for fragmentation during the procedure, operation time from the start to the end of the procedure in minutes, hospitalization time from the end of the procedure to the discharge in days, and fluoroscopy time (FT) from the beginning to the end of the procedure is stated in minutes as secondary outcome measures.
Statistical analysis
Statistical analysis was performed with SPSS 25.0 (Statistical Package). Categorical variables are described by frequencies and percentages. Continuous variables are presented as mean and standard deviations. Independent T, Kruskal–Wallis and Chi-square (χ2) tests were used to compare the relationship between categorical and continuous variables subgroups. A P value below 0.05 was considered statistically significant. Propensity Score matching was used to homogenize the groups in the study.
Results
Ninety six patients were determined in both groups, and then the analysis was made. There was no difference between the groups regarding age, gender, stone side, and stone location (Table 1). In Group 1, 21 (21.8%) patients had lower pole stones, while 20 (20.8%) had multiple calyceal stones. In Group 2, 19 (19.8%) patients had lower pole stones, while 24 (25.1%) patients had stones in multiple calyces (p = 0.118).
While dusting, fragmentation, and popcorn techniques were used less alone in Group 1, it was observed that the combined technique was preferred more than Group 2 (p < 0.001). When the combined fragmentation technique was compared, it was used in 82 (85.4%) patients in Group 1 and 39 (39.6%) patients in Group 2. Stone fragmentation was observed in 2 (2.1%) patients in both groups.In the examination of the laser settings used; the mean frequency value used was 10.3 in Group 1, the mean was 37.9 in Group 2; the average energy level used was 1.8 J in Group 1; It was observed that the mean was 0.5 J in Group2 (p < 0.001).
Stone-free rate: While the stone-free rate on the first day was 80.3% in Group 1, it was 78.1% in Group 2 (p = 0.9). In the third month, it was 90.7% in Group 1 and 87.5% in Group 2; there was no significant difference between the groups (p:0.7). The preoperative serum creatinine value was 0.98 ± 0.38 mg/dl in Group 1 and 1.09 ± 0.58 mg/dl in Group 2, the postoperative serum creatinine value was 0.95 ± 0.32 mg/dl in Group 1 and 1.02 ± 0.37 mg/dl in Group 2, and there was no difference between the groups (respectively; p = 0.6; p = 0.3) (Table 2).
Hospitalization time: Hospitalization duration was significantly higher in Group 1. (2.35 ± 2.27 days vs. 1.42 ± 1.10 days; p < 0.001).
Operation time: The operation duration was 88.70 ± 29.72 min in Group1 and 66.17 ± 41.02 min in Group2 (p < 0.001). The FT was 90.73 ± 4.79 s in Group 1 and 50.78 ± 5.64 s in Group 2 (p < 0.001) (Table 2). The correlation analysis found a positive correlation between the operation duration, fragmentation time, FT, and hospitalization (p < 0.001) (Table 2).
Complications: Auxiliary procedure requirements, complications according to Clavien Classification, UAS usage, and postoperative stent placement were similar between the groups(p > 0.05) (Table 2).
Discussion
The lasers’ power in treating urinary tract stones ranges from 10 to 140 watts (W). In research and clinical practice, lasers > 35 W are accepted as HPL and those with < 35 W as LPL [3, 4]. While low-power holmium lasers can be used for lithotripsy and some other urological interventions except prostate resection, they offer advantages with lower purchasing costs. HPL, on the other hand, is claimed to provide faster disintegration and minimize the need for baskets when removing stones during lithotripsy. Apart from stones, it is also used in various reconstructive surgical procedures with endoscopic prostate intervention.
Our study found similar SFR and complication rates with HPL and LPL. In addition, patients who used HPL had lower operation time, hospital stay, and FT than the LPL group. Previous studies comparing HPL and LPL reported similar SFR at both laser powers [5,6,7]. Furthermore, SFR was similar for the first and postoperative third months at different energies.
Pietropaolo et al. reported operation durations of 52.02 ± 27.90 min in patients who used LPL; and 38.46 ± 22.88 min in patients using HPL (p < 0.001)[5]. Another study by Shrestha et al. reported 38 (19–60) min in the LPL group and 40 (25–60) min in the HPL group [6]. Moreover, Golomb et al. reported the mean operation duration as 53 (15–168) min [8]. However, stone sizes and measurement techniques vary widely between studies. In our study, the operation time in the LPL group was 88.7 ± 29.5 min and 66.1 ± 41 min in the HPL group. We think the longer surgery duration in our study may be due to the larger stone diameter and higher cumulative mean stone length.
The relationship between FURS complications, operative times, and the thermal effect of the laser on the tissue has been reported previously [9, 10]. Complications increase by prolonged operation time and increased intrarenal pressure [10, 11]. Our study reports postoperative complications according to the Clavien-Dindo grading [11, 12]. In the literature comparing HPL and LPL as complications, the complication rates were changing between 4.3–21% vs. 4.7–17.7% for HPL and LPL, respectively (5,6,8); it was 22.9–18.2% for HPL and LPL in our study, and no difference was found in terms of complications between the groups. Although serum creatinine measurement is nonspecific, it indirectly indicates tubular damage, and preoperative and postoperative creatinine values and kidney damage markers were considered [13, 14]. In the study of Ertas et al., the preoperative serum creatinine value was 0.97 ± 0.58 mg/dl, while the post-op serum creatinine value was 1.0 ± 0.61 mg/dl [14]. A study found that the serum creatinine level, which was 0.89 ± 0.22 preoperatively, increased to 0.98 ± 0.25 in the postoperative measurement in patients who underwent RIRS using UAS [13, 14]. We did not determine a significant difference between the HPL and LPL groups; however, further studies with more specific kidney damage markers are needed to clarify the effect of different energy sources and power settings on kidney functions.
Sfoungaristos et al., evaluating fluoroscopy times on patients who underwent URS and RIRS, reported that the mean fluoroscopy times were 41.4 s for a more experienced surgeon and 91 s and 44.9 s for two junior surgeons. The procedure time, postoperative double-J stent use, and less surgical experience were independent predictors of increased FT [15]. In our study, the duration of FT in the LPL group was higher than in the HPL group, which can be due to the increased operation time in the LPL group.
Limitations of the study
The retrospective design and the fact that the interventions were performed in different centers and by different surgeons is a significant limitation of our study. Therefore, propensity matching was done to homogenize the groups. Also, a significant limitation was using different lasers in different settings in both LPL and HPL. The absence of a standardized lithotripsy setting resulted in heterogeneity between groups. Similarly, measuring blood biochemistry in different centers may make a difference when evaluating the data. This study was designed retrospectively, and homogenization between groups was provided by propensity score analysis. In this study, we showed the effects of different power lasers on the success of RIRS. However, more prospectively designed, multicentric studies needed to be conducted in selected patient groups to determine the optimal energy sources and settings.
Conclusion
It is seen that HPL and LPL are used with similar success and complication rates, and the duration of operation, hospitalization, and FT is reduced with HPL. Studies using properly designed and appropriate biomarkers will better understand how it affects kidney functions in the medium and long term. Although high-power lasers are expensive in terms of cost, they affect many parameters and strengthen the hand of urologists thanks to the wide energy and frequency range they offer.
References
Türk C et al (2016) EAU guidelines on interventional treatment for urolithiasis. Euro Urol 69(3):475–482
Assimos D et al (2016) Surgical management of stones: American urological association/endourological society guideline PART I. J Urol 196(4):1153–1160
Turk C, Petrik A, Sarica K (2015) EAU guidelines on urolithiasis. Eur Assoc Urol 69:475–482
Basulto-Martínez M et al (2020) Holmium laser for RIRS Watts are we doing? Arch Esp Urol 73(8):735–744
Pietropaolo A et al (2022) Role of low-versus high-power laser in the treatment of lower pole stones: prospective non-randomized outcomes from a university teaching hospital. Ther Adv Urol 14:17562872221097344
Shrestha A et al (2022) Comparison of low power and high power holmium YAG laser settings in flexible ureteroscopy. World J Urol 40:1839–1844
Kourambas J, Delvecchio FC, Preminger GM (2001) Low-power holmium laser for the management of urinary tract calculi, strictures, and tumors. J Endourol 15(5):529–532
Golomb D (2023) Retrograde intrarenal surgery for lower pole stones utilizing stone displacement technique yields excellent results. Asian J Urol 10(1):58–63
Aldoukhi AH et al (2018) Understanding the popcorn effect during holmium laser lithotripsy for dusting. Urol 122:52–57
Whitehurst L et al (2020) Factors affecting operative time during ureteroscopy and stone treatment and its effect on outcomes: retrospective results over 6.5 years. Ther Adv Urol 12:1756287220934403
Pietropaolo A et al (2021) A machine learning predictive model for post-ureteroscopy urosepsis needing intensive care unit admission: a case–control yau endourology study from nine european centres. J Clin Med 10(17):3888
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205
Fried L (2020) When increase in serum creatinine doesn’t imply kidney damage. Am Soc Nephrol 15:304–305
Gökhan E et al (2022) Comparison of retrograde intrarenal stone surgery with and without a ureteral access sheath using kidney injury molecule-1 (KIM-1) levels: a prospective randomized study. Urolithiasis 50:625–633
Sfoungaristos S et al (2015) Surgical experience gained during an endourology fellowship program may affect fluoroscopy time during ureterorenoscopy. Urolithiasis 43:369–374
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
E.E., G.E.,S.G., K.A. ans K.S. wrote the main manuscript text, and prepared figure and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no confict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erol, E., Ecer, G., Kiremit, M.C. et al. Multicentric evaluation of high and low power lasers on RIRS success using propensity score analysis. Urolithiasis 52, 32 (2024). https://doi.org/10.1007/s00240-024-01535-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-024-01535-w