Abstract
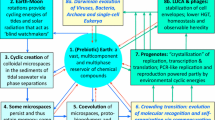
For the RNA-world hypothesis to be ecologically feasible, selection mechanisms acting on replicator communities need to be invoked and the corresponding scenarios of molecular evolution specified. Complementing our previous models of chemical evolution on mineral surfaces, in which selection was the consequence of the limited mobility of macromolecules attached to the surface, here we offer an alternative realization of prebiotic group-level selection: the physical encapsulation of local replicator communities into the pores of the mineral substrate. Based on cellular automaton simulations we argue that the effect of group selection in a mineral honeycomb could have been efficient enough to keep prebiotic ribozymes of different specificities and replication rates coexistent, and their metabolic cooperation protected from extensive molecular parasitism. We suggest that mutants of the mild parasites persistent in the metabolic system can acquire useful functions such as replicase activity or the production of membrane components, thus opening the way for the evolution of the first autonomous protocells on Earth.












Similar content being viewed by others
References
Baaske P, Weinert FM, Duhr S, Lemke KH, Russell MJ, Braun D (2007) Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc Natl Acad Sci 104:9346–9351
Bernal JD (1951) The physical basis of life. Routledge and Paul, London
Biondi E, Branciamore S, Fusi L, Gago S, Gallori E (2007a) Catalytic activity of hammerhead ribozymes in a clay mineral environment: implications for the RNA world. Gene 389:10–18
Biondi E, Branciamore S, Maurel MC, Gallori E (2007b) Montmorillonite protection of an UV-irradiated hairpin ribozyme: evolution of the RNA world in a mineral environment. BMC Evol Biol 7(Suppl 2):S2
Costanzo G, Saladino R, Crestini C, Ciciriello F, Di Mauro E (2007) Formamide as the main building block in the origin of nucleic acids. BMC Evol Biol 7. doi:10.1186/1471-2148-7-S2-S1
Czárán T, Szathmáry E (2000) Coexistence of replicators in prebiotic evolution. In: Dieckmann U, Law R, Metz JAJ (eds) The geometry of ecological interactions: simplifying spatial complexity. Cambridge University Press, Cambridge, pp 116–134
Eigen M, Schuster P (1977) A principle of natural self-organization. Naturwissenschaften 64:541–565
Eigen M, Schuster P (1979) The abstract hypercycle. Naturwissenschaften 66:512–512
Ertem G, Ferris JP (1996) Synthesis of RNA oligomers on heterogeneous templates. Nature 379:238–240
Ferris JP, Hill AR, Liu RH, Orgel LE (1996) Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381:59–61
Fontanari JF, Santos M, Szathmáry E (2006) Coexistence and error propagation in pre-biotic vesicle models: a group selection approach. J Theor Biol 239:247–256
Gallori E, Biondi E, Branciamore S (2006) Looking for the primordial genetic honeycomb. Origins Life Evol Biosph 36:493–499
Gánti T (2003) Chemoton theory: theory of living systems. Oxford University Press, Oxford
Gilbert W (1986) Origin of life: the RNA world. Nature 319:618–618
Greenberg JM, Hastings SP (1978) Spatial patterns for discrete models of diffusion in excitable media. SIAM J Appl Math 34:515–523
Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35:849–857
Joyce GF (2002) The antiquity of RNA-based evolution. Nature 418:214–221
Könnyü B, Czárán T, Szathmáry E (2008) Prebiotic replicase evolution in a surface-bound metabolic system: parasites as a source of adaptive evolution. BMC Evol Biol 8. doi:10.1186/1471-2148-8-267
Koonin EV (2007) An RNA-making reactor for the origin of life. Proc Natl Acad Sci 104:9105–9106
Koonin EV, Martin W (2005) On the origin of genomes and cells within inorganic compartments. Trends Genet 21:647–654
Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR (1982) Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of tetrahymena. Cell 31:147–157
Lincoln TA, Joyce GF (2009) Self-sustained replication of an RNA enzyme. Science 323:1229–1232
Martin W, Russell MJ (2003) On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos Trans Roy Soc Lond Ser B Biol Sci 358:59–83
Martin W, Russell MJ (2007) On the origin of biochemistry at an alkaline hydrothermal vent. Philos Trans Roy Soc B Biol Sci 362:1887–1925
Maynard Smith J (1979) Hypercycles and the origin of life. Nature 280:445–446
Maynard Smith J, Szathmáry E (1995) The major transitions in evolution. Freeman, Oxford
Moore PB, Steitz TA (2002) The involvement of RNA in ribosome function. Nature 418:229–235
Orgel LE (2004) Prebiotic chemistry and the origin of the RNA world. Crit Rev Biochem Mol Biol 39:99–123
Parsons I, Lee MR, Smith JV (1998) Biochemical evolution II: origin of life in tubular microstructures on weathered feldspar surfaces. Proc Natl Acad Sci USA 95:15173–15176
Ricardo A, Carrigan MA, Olcott AN, Benner SA (2004) Borate minerals stabilize ribose. Science 303:196–196
Saladino R, Crestini C, Ciambecchini U, Ciciriello F, Costanzo G, Di Mauro E (2004) Synthesis and degradation of nucleobases and nucleic acids by formamide in the presence of montmorillonites. Chembiochem 5:1558–1566
Scheuring I, Czárán T, Szabo P, Karolyi G, Toroczkai Z (2003) Spatial models of prebiotic evolution: soup before pizza? Origins Life Evol Biosph 33:319–355
Smith JV (1998) Biochemical evolution. I. Polymerization on internal, organophilic silica surfaces of dealuminated zeolites and feldspars. Proc Natl Acad Sci USA 95:3370–3375
Smith JV, Arnold FP, Parsons I, Lee MR (1999) Biochemical evolution III: polymerization on organophilic silica-rich surfaces, crystal-chemical modeling, formation of first cells, and geological clues. Proc Natl Acad Sci USA 96:3479–3485
Steitz TA, Moore PB (2003) RNA, the first macromolecular catalyst: the ribosome is a ribozyme. Trends Biochem Sci 28:411–418
Szathmáry E (2006) The origin of replicators and reproducers. Philos Trans Roy Soc B Biol Sci 361:1761–1776
Szathmáry E (2007) Coevolution of metabolic networks and membranes: the scenario of progressive sequestration. Philos Trans Roy Soc B Biol Sci 362:1781–1787
Szathmáry E, Demeter L (1987) Group selection of early replicators and the origin of life. J Theor Biol 128:463–486
Wächtershäuser G (1992) Groundworks for an evolutionary biochemistry: the iron–sulphur world. Prog Biophys Mol Biol 58:85–201
Zintzaras E, Santos M, Szathmary E (2002) “Living” under the challenge of information decay: the stochastic corrector model vs. hypercycles. J Theor Biol 217(2):167–181
Acknowledgements
We thank W. de Back for useful discussion. Part of this work was supported by the Italian Space Agency, ASI-ESS Project. Financial support by the Hungarian Scientific Research Fund (OTKA Grant No. K-67907) for T.C. is acknowledged. E.S. is supported by the National Office for Research and Technology (NAP 2005/KCKHA005). Support by COST CM0703 (Systems chemistry) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Branciamore, S., Gallori, E., Szathmáry, E. et al. The Origin of Life: Chemical Evolution of a Metabolic System in a Mineral Honeycomb?. J Mol Evol 69, 458–469 (2009). https://doi.org/10.1007/s00239-009-9278-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-009-9278-6