Abstract
Purpose
To use structural magnetic resonance imaging and graph theory approaches to investigate the topological organization of the brain morphological network based on gray matter in essential tremor, and its potential relation to disease severity.
Methods
In this prospective study conducted from November 2018 to November 2019, 36 participants with essential tremor and 37 matched healthy controls underwent magnetic resonance imaging. Brain networks based on the morphological similarity of gray matter across regions were analyzed using graph theory. Nonparametric permutation testing was used to assess group differences in topological metrics. Support vector machine was applied to the gray matter morphological matrices to classify participants with essential tremor vs. healthy controls.
Results
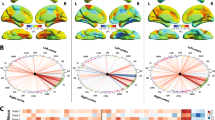
Compared with healthy controls, participants with essential tremor showed increased global efficiency (p < 0.01) and decreased path length (p < 0.01); abnormal nodal properties in frontal, parietal, and cerebellar lobes; and disconnectivity in cerebello-thalamo-cortical network. The abnormal brain nodal centralities (left superior cerebellum gyrus; right caudate nucleus) correlated with clinical measures, both motor (Fahn–Tolosa–Marìn tremor rating, p = 0.017, r = − 0.41) and nonmotor (Hamilton depression scale, p = 0.040, r = − 0.36; Hamilton anxiety scale, p = 0.008, r = − 0.436). Gray matter morphological matrices classified individuals with high accuracy of 80.0%.
Conclusion
Participants with essential tremor showed randomization in global properties and dysconnectivity in the cerebello-thalamo-cortical network. Participants with essential tremor could be distinguished from healthy controls by gray matter morphological matrices.



Similar content being viewed by others
References
Malkki H (2016) Movement disorders: novel genetic risk variants for essential tremor. Nat Rev Neurol 12(12):679
Hopfner F, Ahlf A, Lorenz D, Klebe S, Zeuner KE, Kuhlenbaumer G, Deuschl G (2016) Early- and late-onset essential tremor patients represent clinically distinct subgroups. Mov Disord 31(10):1560–1566
Benito-Leon J, Louis ED (2006) Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol 2(12):666–678
Benito-Leon J, Sanz-Morales E, Melero H, Louis ED, Romero JP, Rocon E, Malpica N (2019) Graph theory analysis of resting-state functional magnetic resonance imaging in essential tremor. Hum Brain Mapp 40(16):4686–4702
Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Louis ED (2009) Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. J Neurol Sci 287(1-2):138–142
Buijink AW, van der Stouwe AM, Broersma M, Sharifi S, Groot PF, Speelman JD, Maurits NM, van Rootselaar AF (2015) Motor network disruption in essential tremor: a functional and effective connectivity study. Brain 138(Pt 10):2934–2947
Gong Q (2020) Psychoradiology. Neuroimaging Clinics of North America. Elsevier Inc, New York
Suo X, Lei D, Li N, Cheng L, Chen F, Wang M, Kemp GJ, Peng R, Gong Q (2017) Functional brain connectome and its relation to Hoehn and Yahr stage in Parkinson disease. Radiology 285(3):904–913
Port JD (2018) Diagnosis of attention deficit hyperactivity disorder by using MR imaging and radiomics: a potential tool for clinicians. Radiology 287(2):631–2
Sun H, Chen Y, Huang Q, Lui S, Huang X, Shi Y, Xu X, Sweeney JA, Gong Q (2018) Psychoradiologic Utility of MR Imaging for Diagnosis of Attention Deficit Hyperactivity Disorder: A Radiomics Analysis. Radiology 287(2):620–630
He Y, Chen ZJ, Evans AC (2007) Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17(10):2407–2419
Wang H, Jin X, Zhang Y, Wang J (2016) Single-subject morphological brain networks: connectivity mapping, topological characterization and test-retest reliability. Brain Behav 6(4):e00448
Van Essen DC (1997) A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385(6614):313–318
Hilgetag CC, Barbas H (2005) Developmental mechanics of the primate cerebral cortex. Anat Embryol (Berl) 210(5-6):411–417
Alexander-Bloch A, Giedd JN, Bullmore E (2013) Imaging structural co-variance between human brain regions. Nat Rev Neurosci 14(5):322–336
Evans AC (2013) Networks of anatomical covariance. Neuroimage 80:489–504
Kong XZ, Liu Z, Huang L, Wang X, Yang Z, Zhou G, Zhen Z, Liu J (2015) Mapping individual brain networks using statistical similarity in regional morphology from MRI. PLoS One 10(11):e0141840
Kong XZ, Wang X, Huang L, Pu Y, Yang Z, Dang X, Zhen Z, Liu J (2014) Measuring individual morphological relationship of cortical regions. J Neurosci Methods 237:103–107
Homan P, Argyelan M, DeRosse P, Szeszko PR, Gallego JA, Hanna L, Robinson DG, Kane JM, Lencz T, Malhotra AK (2019) Structural similarity networks predict clinical outcome in early-phase psychosis. Neuropsychopharmacology 44(5):915–922
Li X, Lei D, Niu R, Li L, Suo X, Li W, Yang C, Yang T, Ren J, Pinaya WHL, Zhou D, Kemp GJ, Gong Q (2020) Disruption of gray matter morphological networks in patients with paroxysmal kinesigenic dyskinesia. Hum Brain Mapp 42(2):398–411
Benito-Leon J, Louis ED, Romero JP, Hernandez-Tamames JA, Manzanedo E, Alvarez-Linera J, Bermejo-Pareja F, Posada I, Rocon E (2015) Altered functional connectivity in essential tremor: a resting-state fMRI study. Medicine (Baltimore) 94(49):e1936
Chen T, Kendrick KM, Wang J, Wu M, Li K, Huang X, Luo Y, Lui S, Sweeney JA, Gong Q (2017) Anomalous single-subject based morphological cortical networks in drug-naive, first-episode major depressive disorder. Hum Brain Mapp 38(5):2482–2494
Deuschl G, Bain P, Brin M, Ad Hoc Sci C (1998) Consensus statement of the Movement Disorder Society on tremor. Mov Disord 13:2–23
Koller WC (1990) Parkinson’s disease and movement disorders, Edited by J. Jankwic and E. Tolosa Baltimore, Urban and Schwarzenberg, 1988 499 pp, illustrated. 27 (4):452–453
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851
Wen W, Zhu W, He Y, Kochan NA, Reppermund S, Slavin MJ, Brodaty H, Crawford J, Xia A, Sachdev P (2011) Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J Neurosci 31(4):1204–1212
Watts DJ, Strogatz SH (1998) Collective dynamics of ‘small-world’ networks. Nature 393(6684):440–442
Wang J, Wang X, Xia M, Liao X, Evans A, He Y (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9:386
Zhang W, Lei D, Keedy SK, Ivleva EI, Eum S, Yao L, Tamminga CA, Clementz BA, Keshavan MS, Pearlson GD, Gershon ES, Bishop JR, Gong Q, Lui S, Sweeney JA (2020) Brain gray matter network organization in psychotic disorders. Neuropsychopharmacology 45(4):666–674
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–69
Latora V, Marchiori M (2001) Efficient behavior of small-world networks. Phys Rev Lett 87(19):198701
Filippi M, van den Heuvel MP, Fornito A, He Y, Hulshoff Pol HE, Agosta F, Comi G, Rocca MA (2013) Assessment of system dysfunction in the brain through MRI-based connectomics. Lancet Neurol 12(12):1189–1199
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2):e17
Zalesky A, Fornito A, Bullmore ET (2010) Network-based statistic: identifying differences in brain networks. Neuroimage 53(4):1197–1207
He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, Evans A (2009) Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain 132(Pt 12):3366–3379
Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, Gong Q (2011) Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry 70(4):334–342
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15(4):870–878
Chang CC, Lin CJ (2011) LIBSVM: A library for support vector machines. ACM Trans Intell Syst Technol 2(3):1–27
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V (2012) Scikit-learn: machine learning in Python. J Mach Learn Res 12(10):2825–2830
Tijms BM, Moller C, Vrenken H, Wink AM, de Haan W, van der Flier WM, Stam CJ, Scheltens P, Barkhof F (2013) Single-subject grey matter graphs in Alzheimer’s disease. PLoS One 8(3):e58921
Niu R, Lei D, Chen F, Chen Y, Suo X, Li L, Lui S, Huang X, Sweeney JA, Gong Q (2018) Reduced local segregation of single-subject gray matter networks in adult PTSD. Hum Brain Mapp 39(12):4884–4892
Lenka A, Bhalsing KS, Panda R, Jhunjhunwala K, Naduthota RM, Saini J, Bharath RD, Yadav R, Pal PK (2017) Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor. Neuroradiology 59(2):157–168
Louis ED (2016) De Sedibus et causis morborum: is essential tremor a primary disease of the cerebellum? Cerebellum 15(3):233–234
Louis ED, Rabinowitz D, Choe M, Tate WJ, Kelly GC, Kuo SH, Faust PL (2016) Mapping Purkinje cell placement along the purkinje cell layer: an analysis of postmortem tissue from essential tremor patients vs. controls. Cerebellum 15(6):726–731
Fang W, Chen H, Wang H, Zhang H, Puneet M, Liu M, Lv F, Luo T, Cheng O, Wang X, Lu X (2016) Essential tremor is associated with disruption of functional connectivity in the ventral intermediate Nucleus--Motor Cortex--Cerebellum circuit. Hum Brain Mapp 37(1):165–178
Louis ED, Huang CC, Dyke JP, Long Z, Dydak U (2014) Neuroimaging studies of essential tremor: how well do these studies support/refute the neurodegenerative hypothesis? Tremor Other Hyperkinet Mov (N Y) 4:235
Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G (2001) The gait disorder of advanced essential tremor. Brain 124(Pt 11):2278–2286
Price JL, Drevets WC (2012) Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci 16(1):61–71
Vriend C, Raijmakers P, Veltman DJ, van Dijk KD, van der Werf YD, Foncke EM, Smit JH, Berendse HW, van den Heuvel OA (2014) Depressive symptoms in Parkinson’s disease are related to reduced [123I]FP-CIT binding in the caudate nucleus. J Neurol Neurosurg Psychiatry 85(2):159–164
Kim MJ, Hamilton JP, Gotlib IH (2008) Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res 164(2):114–122
Louis ED, Benito-León J, Bermejo-Pareja F (2007) Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol 14(10):1138–1146
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198
Lei D, Pinaya WHL, van Amelsvoort T, Marcelis M, Donohoe G, Mothersill DO, Corvin A, Gill M, Vieira S, Huang X, Lui S, Scarpazza C, Young J, Arango C, Bullmore E, Gong Q, McGuire P, Mechelli A (2019) Detecting schizophrenia at the level of the individual: relative diagnostic value of whole-brain images, connectome-wide functional connectivity and graph-based metrics. Psychol Med 50(11):1852–1861
Camchong J, MacDonald AW 3rd, Bell C, Mueller BA, Lim KO (2011) Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull 37(3):640–650
Passamonti L, Cerasa A, Quattrone A (2012) Neuroimaging of essential tremor: what is the evidence for cerebellar involvement? Tremor Other Hyperkinet Mov (NY) 2:02–67–421–3. https://doi.org/10.7916/D8F76B8G
Mechelli A, Prata D, Kefford C, Kapur S (2015) Predicting clinical response in people at ultra-high risk of psychosis: a systematic and quantitative review. Drug Discov Today 20(8):924–927
Wong A, Xiong YY, Kwan PW, Chan AY, Lam WW, Wang K, Chu WC, Nyenhuis DL, Nasreddine Z, Wong LK, Mok VC (2009) The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement Geriatr Cogn Disord 28(1):81–87
Nasreddine ZS, Phillips N, Chertkow H (2012) Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology 78(10):765–766
Acknowledgements
The authors would like to thank the families who participated in this study.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Nos. 81621003, 82027808) and the Functional and Molecular Imaging Key Laboratory of Sichuan Province (FMIKLSP, Grant: 2019JDS0044).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 409 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Lei, D., Peng, J. et al. Disrupted brain gray matter networks in drug-naïve participants with essential tremor. Neuroradiology 63, 1501–1510 (2021). https://doi.org/10.1007/s00234-021-02653-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02653-7