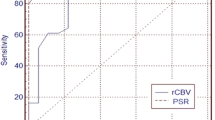
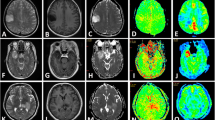
Abstract
Purpose
MRI is a useful method for discriminating low- and high-grade glioma using perfusion MRI and susceptibility-weighted imaging (SWI). The purpose of this study is to evaluate the usefulness of T1-perfusion MRI and SWI in discriminating among grade II, III, and IV gliomas.
Methods
T1-perfusion MRI was used to measure relative cerebral blood volume (rCBV) in 129 patients with glioma (70 grade IV, 33 grade III, and 26 grade II tumors). SWI was also used to measure the intratumoral susceptibility signal intensity (ITSS) scores for each tumor in these patients. rCBV and ITSS values were compared to seek differences between grade II vs. grade III, grade III vs. grade IV, and grade III+II vs. grade IV tumors.
Results
Significant differences in rCBV values of the three grades of the tumors were noted and pairwise comparisons showed significantly higher rCBV values in grade IV tumors as compared to grade III tumors, and similarly increased rCBV was seen in the grade III tumors as compared to grade II tumors (p < 0.001). Grade IV gliomas showed significantly higher ITSS scores on SWI as compared to grade III tumors (p < 0.001) whereas insignificant difference was seen on comparing ITSS scores of grade III with grade II tumors. Combining the rCBV and ITSS resulted in significant improvement in the discrimination of grade III from grade IV tumors.
Conclusion
The combination of rCBV values derived from T1-perfusion MRI and SWI derived ITSS scores improves the diagnostic accuracy for discrimination of grade III from grade IV gliomas.

Similar content being viewed by others
References
Law M, Oh S, Babb JS et al (2006) Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. J Magn Reson Imaging 39:1569–1574
McLendon RE, Halperin EC (2003) Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer 98:1745–1748
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–410
Jain R, Ellika SK, Scarpace L et al (2008) Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. AJNR Am J Neuroradiol 29:694–700
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Shin JH, Lee HK, Kwun BD et al (2002) Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: preliminary results. AJR Am J Roentgenol 179:783–789
Roy B, Gupta RK, Maudsley AA et al (2013) Utility of multiparametric 3-T MRI for glioma characterization. Neuroradiology 55:603–613
Park MJ, Kim HS, Jahng GH, Ryu CW, Park SM, Kim SY (2009) Semiquantitative assessment of intratumoral susceptibility signals using non-contrast-enhanced high-field high-resolution susceptibility-weighted imaging in patients with gliomas: comparison with MR perfusion imaging. AJNR Am J Neuroradiol 30:1402–1408
Wang X, Zhang H, Tan Y et al (2014) Combined value of susceptibility-weighted and perfusion-weighted imaging in assessing WHO grade for brain astrocytomas: combined SWI and DSC in brain astrocytomas. J Magn Reson Imaging 39:1569–1574
Li X, Zhu Y, Kang H et al (2015) Glioma grading by microvascular permeability parameters derived from dynamic contrast-enhanced MRI and intratumoral susceptibility signal on susceptibility weighted imaging. Cancer Imaging 15:4
Ohgaki H, Kleihues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64:479–489
Le Rhun E, Taillibert S, Chamberlain MC (2016) Current management of adult diffuse infiltrative low grade gliomas. Curr Neurol Neurosci Rep 16:15
Wang Y, Jiang T (2013) Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett 331:139–146
Collet S, Valable S, Constans JM et al (2015) [(18)F]-fluoro-L-thymidine PET and advanced MRI for preoperative grading of gliomas. Neuroimage Clin 8:448–454
Wiestler B, Kluge A, Lukas M et al (2016) Multiparametric MRI-based differentiation of WHO grade II/III glioma and WHO grade IV glioblastoma. Sci Rep 6:35142
Falk A, Fahlström M, Rostrup E et al (2014) Discrimination between glioma grades II and III in suspected low-grade gliomas using dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging: a histogram analysis approach. Neuroradiology 56:1031–1038
Singh A, Haris M, Rathore D et al (2007) Quantification of physiological and hemodynamic indices using T(1) dynamic contrast-enhanced MRI in intracranial mass lesions. J Magn Reson Imaging 26:871–880
Sahoo P, Rathore RKS, Awasthi R et al (2013) Subcompartmentalization of extracellular extravascular space (EES) into permeability and leaky space with local arterial input function (AIF) results in improved discrimination between high- and low-grade glioma using dynamic contrast-enhanced (DCE) MRI. J Magn Reson Imaging 38:677–688
Cha S, Johnson G, Wadghiri YZ et al (2003) Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: correlation with histopathology. Magn Reson Med 49:848–855
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Lacerda S, Law M (2009) Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am 19:527–557
Sugahara T, Korogi Y, Kochi M et al (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 171:1479–1486
Sahoo P, Gupta RK, Gupta PK et al (2017) Diagnostic accuracy of automatic normalization of CBV in glioma grading using T1- weighted DCE-MRI. Magn Reson Imaging 44:32–37
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Robin X, Turck N, Hainard A et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77
Hirai T, Murakami R, Nakamura H et al (2008) Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol 29:1505–1510
Zonari P, Baraldi P, Crisi G (2007) Multimodal MRI in the characterization of glial neoplasms: the combined role of single-voxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging. Neuroradiology 49:795–803
Chan AS, Leung SY, Wong MP et al (1998) Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol 22:816–826
Lev MH, Ozsunar Y, Henson JW et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol 25:214–221
Furtner J, Schöpf V, Preusser M et al (2014) Non-invasive assessment of intratumoral vascularity using arterial spin labeling: a comparison to susceptibility-weighted imaging for the differentiation of primary cerebral lymphoma and glioblastoma. Eur J Radiol 83:806–810
Li C, Ai B, Li Y, Qi H, Wu L (2010) Susceptibility-weighted imaging in grading brain astrocytomas. Eur J Radiol 75:e81–e85
Pinker K, Noebauer-Huhmann IM, Stavrou I et al (2007) High-resolution contrast-enhanced, susceptibility-weighted MR imaging at 3T in patients with brain tumors: correlation with positron-emission tomography and histopathologic findings. AJNR Am J Neuroradiol 28:1280–1286
van den Bent MJ (2010) Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol 120:297–304
Christoforidis GA, Kangarlu A, Abduljalil AM et al (2004) Susceptibility-based imaging of glioblastoma microvascularity at 8 T: correlation of MR imaging and postmortem pathology. AJNR Am J Neuroradiol 25:756–760
Reuss DE, Mamatjan Y, Schrimpf D et al (2015) IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol 129:867–873
Hartmann C, Hentschel B, Wick W et al (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120:707–718
Lee S, Choi SH, Ryoo I et al (2015) Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J Neuro-Oncol 121:141–150
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We declare that the study has been approved by the ethics committee of the Fortis Memorial Research Institute, Gurgaon, India, and National Institute of Mental Health and Neurosciences, Bangalore, India, and have therefore been performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Saini, J., Gupta, P.K., Sahoo, P. et al. Differentiation of grade II/III and grade IV glioma by combining “T1 contrast-enhanced brain perfusion imaging” and susceptibility-weighted quantitative imaging. Neuroradiology 60, 43–50 (2018). https://doi.org/10.1007/s00234-017-1942-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1942-8