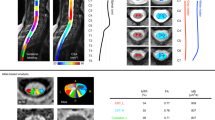
Abstract
Purpose
The aim of this prospective study was to determine the feasibility in terms of repeatability and reproducibility of diffusional kurtosis imaging (DKI) for microstructural assessment of the normal cervical spinal cord (cSC) using a phase-sensitive inversion recovery (PSIR) sequence as the anatomical reference for accurately defining white-matter (WM) and gray-matter (GM) regions of interests (ROIs).
Methods
Thirteen young healthy subjects were enrolled to undergo DKI and PSIR sequences in the cSC. The repeatability and reproducibility of kurtosis metrics and fractional anisotropy (FA) were calculated in GM, WM, and cerebral-spinal-fluid (CSF) ROIs drawn by two independent readers on PSIR images of three different levels (C1–C4). The presence of statistically significant differences in DKI metrics for levels, ROIs (GM, WM, and CSF) repeatability, reproducibility, and inter-reader agreement was evaluated.
Results
Intra-class correlation coefficients between the two readers ranged from good to excellent (0.75 to 0.90). The inferior level consistently had the highest concordance. The lower values of scan–rescan variability for all DKI parameters were found for the inferior level. Statistically significant differences in kurtosis values were not found in the lateral white-matter bundles of the spinal cord.
Conclusion
The integration of DKI and PSIR sequences in a clinical MR acquisition to explore the regional microstructure of the cSC in healthy subjects is feasible, and the results obtainable are reproducible. Further investigation will be required to verify the possibility to translate this method to a clinical setting to study patients with SC involvement especially in the absence of MRI abnormalities on standard sequences.




Similar content being viewed by others
References
Andre JB, Bammer R (2010) Advanced diffusion-weighted magnetic resonance imaging techniques of the human spinal cord. Top Magn Reson Imaging 21(6):367–378. doi:10.1097/RMR.0b013e31823e65a1
Samson RS, Lévy S, Schneider T, Smith AK, Smith SA, Cohen-Adad J, Wheeler-Kingshott CA (2016) ZOOM or non-ZOOM? Assessing spinal cord diffusion tensor imaging protocols for multi-centre studies. PLoS One 11(5):1–14. doi:10.1371/journal.pone.0155557
Lerner A, Mogensen MA, Kim PE, Shiroishi MS, Hwang DH, Law M (2014) Clinical applications of diffusion tensor imaging. World Neurosurg 82(1–2):96–109. doi:10.1016/j.wneu.2013.07.083
Raz E, Bester M, Sigmund EE, Tabesh A, Babb JS, Jaggi H, Helpern J, Mitnick RJ, Inglese M (2013) A better characterization of spinal cord damage in multiple sclerosis: a diffusional kurtosis imaging study. Am J Neuroradiol 34(9):1846–1852. doi:10.3174/ajnr.A3512
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53(6):1432–1440. doi:10.1002/mrm.20508
Conklin CJ, Middleton DM, Alizadeh M, Finsterbusch J, Raunig DL, Faro SH, Shah P, Krisa L, Sinko R, Delalic JZ, Mulcahey MJ, Mohamed FB (2016) Spatially selective 2D RF inner field of view (iFOV) diffusion kurtosis imaging (DKI) of the pediatric spinal cord. NeuroImage Clin 11:61–67. doi:10.1016/j.nicl.2016.01.009
Hori M, Tsutsumi S, Yasumoto Y, Ito M, Suzuki M, Tanaka FS, Kyogoku S, Nakamura M, Tabuchi T, Fukunaga I, Suzuki Y, Kamagata K, Masutani Y, Aoki S (2014) Cervical spondylosis: evaluation of microstructural changes in spinal cord white matter and gray matter by diffusional kurtosis imaging. Magn Reson Imaging 32(5):428–432. doi:10.1016/j.mri.2014.01.018
Hou P, Hasan KM, Sitton CW, Wolinsky JS, Narayana PA (2005) Phase-sensitive T1 inversion recovery imaging: a time-efficient interleaved technique for improved tissue contrast in neuroimaging. Am J Neuroradiol 26(6):1432–1438
Kearney H, Yiannakas MC, Abdel-Aziz K, Wheeler-Kingshott CA, Altmann DR, Ciccarelli O, Miller DH (2014) Improved MRI quantification of spinal cord atrophy in multiple sclerosis. J Magn Reson Imaging 39(3):617–623. doi:10.1002/jmri.24194
Schraa B. (2013) T1-weighted phase sensitive inversion recovery for imaging multiple sclerosis lesions in the cervical SC. Clinical Neurology MAGNETOM Flash | 5/2013
Papinutto N, Schlaeger R, Panara V, Caverzasi E, Ahn S, Johnson KJ, Zhu AH, Stern WA, Laub G, Hauser SL, Henry RG (2015) 2D phase-sensitive inversion recovery imaging to measure in vivo spinal cord gray and white matter areas in clinically feasible acquisition times. J Magn Reson Imaging 42(3):698–708. doi:10.1002/jmri.24819
Schlaeger R, Papinutto N, Panara V, Bevan C, Lobach IV, Bucci M, Caverzasi E, Gelfand JM, Green AJ, Jordan KM, Stern WA, von Büdingen HC, Waubant E, Zhu AH, Goodin DS, Cree BA, Hauser SL, Henry RG (2014) Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Ann Neurol 76(4):568–580. doi:10.1002/ana.24241
Schlaeger R, Papinutto N, Zhu AH, Lobach IV, Bevan CJ, Bucci M, Castellano A, Gelfand JM, Graves JS, Green AJ, Jordan KM, Keshavan A, Panara V, Stern WA, von Büdingen HC, Waubant E, Goodin DS, Cree BA, Hauser SL, Henry RG (2015) Association between thoracic spinal cord gray matter atrophy and disability in multiple sclerosis. JAMA Neurol 72(8):897–904. doi:10.1001/jamaneurol.2015.0993
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Taber KH, Herrick RC, Weathers SW, Kumar AJ, Schomer DF, Hayman LA (1998) Pitfalls and artifacts encountered in clinical MR imaging of the spine. Radiographics 18(6):1499–1521. doi:10.1148/radiographics.18.6.9821197
Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C (2008) An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging 27(4):425–441. doi:10.1109/TMI.2007.906087
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31(3):1116–1128. doi:10.1016/j.neuroimage.2006.01.015
Tabesh A, Jensen JH, Ardekani BA, Helpern JA (2011) Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med 65(3):823–836. doi:10.1002/mrm.22655
Chang LC, Jones DK, Pierpaoli C (2005) RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 53(5):1088–1095. doi:10.1002/mrm.20426
Bester M, Jensen JH, Babb JS, Tabesh A, Miles L, Herbert J, Grossman RI, Inglese M (2015) Non-Gaussian diffusion MRI of gray matter is associated with cognitive impairment in multiple sclerosis. Mult Scler 21(7):935–944. doi:10.1177/1352458514556295
Helpern JA, Adisetiyo V, Falangola MF, Hu C, Di Martino A, Williams K, Castellanos FX, Jensen JH (2011) Preliminary evidence of altered gray and white matter microstructural development in the frontal lobe of adolescents with attention-deficit hyperactivity disorder: a diffusional kurtosis imaging study. J Magn Reson Imaging 33(1):17–23. doi:10.1002/jmri.22397
Zhu J, Zhuo C, Qin W, Wang D, Ma X, Zhou Y, Yu C (2015) Performances of diffusion kurtosis imaging and diffusion tensor imaging in detecting white matter abnormality in schizophrenia. NeuroImage Clin 7:170–176. doi:10.1016/j.nicl.2014.12.008
Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM (2009) Bayesian analysis of neuroimaging data in FSL. NeuroImage 45(1):S173–S186
De Leener B, Kadoury S, Cohen-Adad J (2014) Robust, accurate and fast automatic segmentation of the spinal cord. NeuroImage 98:528–536. doi:10.1016/j.neuroimage.2014.04.051
Lévy S, Benhamou M, Naaman C, Rainville P, Callot V, Cohen-Adad J (2015) White matter atlas of the human spinal cord with estimation of partial volume effect. NeuroImage 119:262–271. doi:10.1016/j.neuroimage.2015.06.040
Stroman PW, Wheeler-Kingshott C, Bacon M, Schwab JM, Bosma R, Brooks J, Cadotte D, Carlstedt T, Ciccarelli O, Cohen-Adad J, Curt A, Evangelou N, Fehlings MG, Filippi M, Kelley BJ, Kollias S, Mackay A, Porro CA, Smith S, Strittmatter SM, Summers P, Tracey I (2014) The current state-of-the-art of spinal cord imaging: methods. NeuroImage 84:1070–1081. doi:10.1016/j.neuroimage.2013.04.124
Wheeler-Kingshott CA, Stroman PW, Schwab JM, Bacon M, Bosma R, Brooks J, Cadotte DW, Carlstedt T, Ciccarelli O, Cohen-Adad J, Curt A, Evangelou N, Fehlings MG, Filippi M, Kelley BJ, Kollias S, Mackay A, Porro CA, Smith S, Strittmatter SM, Summers P, Thompson AJ, Tracey I (2014) The current state-of-the-art of spinal cord imaging: applications. NeuroImage 84:1082–1093. doi:10.1016/j.neuroimage.2013.07.014
Reich DS, Smith SA, Jones CK, Zackowski KM, van Zijl PC, Calabresi PA, Mori S (2006) Quantitative characterization of the corticospinal tract at 3 Tesla. AJNR Am J Neuroradiol 27(10):2168–2178
Das SK, Wang JL, Bing L, Bhetuwal A, Yang HF (2016) Regional values of diffusional kurtosis estimates in the healthy brain during normal aging. Clin Neuroradiol [Epub ahead of print]. doi:10.1007/s00062-015-0490-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
VP was funded by a grant from the Italian Association of Neuroradiology - AINR.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Panara, V., Navarra, R., Mattei, P.A. et al. Spinal cord microstructure integrating phase-sensitive inversion recovery and diffusional kurtosis imaging. Neuroradiology 59, 819–827 (2017). https://doi.org/10.1007/s00234-017-1864-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1864-5