Abstract
Introduction
We describe the imaging findings encountered in GBM patients receiving immune checkpoint blockade and assess the potential of quantitative MRI biomarkers to differentiate patients who derive therapeutic benefit from those who do not.
Methods
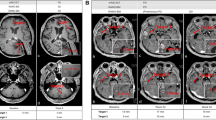
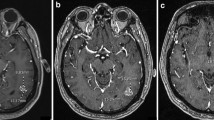
A retrospective analysis was performed on longitudinal MRIs obtained on recurrent GBM patients enrolled on clinical trials. Among 10 patients with analyzable data, bidirectional diameters were measured on contrast enhanced T1 (pGd-T1WI) and volumes of interest (VOI) representing measurable abnormality suggestive of tumor were selected on pGdT1WI (pGdT1 VOI), FLAIR-T2WI (FLAIR VOI), and ADC maps. Intermediate ADC (IADC) VOI represented voxels within the FLAIR VOI having ADC in the range of highly cellular tumor (0.7–1.1 × 10−3 mm2/s) (IADC VOI). Therapeutic benefit was determined by tissue pathology and survival on trial. IADC VOI, pGdT1 VOI, FLAIR VOI, and RANO assessment results were correlated with patient benefit.
Results
Five patients were deemed to have received therapeutic benefit and the other five patients did not. The average time on trial for the benefit group was 194 days, as compared to 81 days for the no benefit group. IADC VOI correlated well with the presence or absence of clinical benefit in 10 patients. Furthermore, pGd VOI, FLAIR VOI, and RANO assessment correlated less well with response.
Conclusion
MRI reveals an initial increase in volumes of abnormal tissue with contrast enhancement, edema, and intermediate ADC suggesting hypercellularity within the first 0–6 months of immunotherapy. Subsequent stabilization and improvement in IADC VOI appear to better predict ultimate therapeutic benefit from these agents than conventional imaging.





Similar content being viewed by others
References
DeAngelis L.M, Wen PY (2012) Primary and metastatic tumors of the nervous system. In: Longo D.L. FAS, Kasper D.L., Hauser S.L., Jameson J, Loscalzo J (eds.) Harrison’s principles of internal medicine
Avril T, Vauleon E, Tanguy-Royer S, Mosser J, Quillien V (2011) Mechanisms of immunomodulation in human glioblastoma. Immunotherapy 3:42–44
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133
Ansell SM, Lesokhin AM, Borrello I et al (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372:311–319
Reardon DA, Gokhale PC, Klein SR et al (2016) Glioblastomaeradication following immune checkpoint blockade in an orthotopic, Immunocompetentmodel. Cancer Immunol Res 4:124–135
Zeng J, See AP, Phallen J et al (2013) Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J RadiatOncolBiolPhys 86:343–349
Wainwright DA, Chang AL, Dey M et al (2014) Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res 20:5290–5301
Reardon DA, Ballman KV, Buckner JC, Chang SM, Ellingson BM (2014) Impact of imaging measurements on response assessment in glioblastoma clinical trials. Neuro-Oncology 16(Suppl 7):vii24–vii35
Okada H, Weller M, Huang R et al (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. The Lancet Oncology 16:e534–e542
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Chu HH, Choi SH, Ryoo I et al (2013) Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology 269:831–840
Vrabec M, Van Cauter S, Himmelreich U et al (2011) MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology 53:721–731
Gupta A, Young RJ, Karimi S et al (2011) Isolated diffusion restriction precedes the development of enhancing tumor in a subset of patients with glioblastoma. AJNR Am J Neuroradiology 32:1301–1306
Wieduwilt MJ, Valles F, Issa S et al (2012) Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res 18:1146–1155
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J ClinOncol 28:1963–1972
Ellingson BM, Kim HJ, Woodworth DC et al (2014) Recurrentglioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology 271:200–210
Porto L, Jurcoane A, Schwabe D, Kieslich M, Hattingen E (2013) Differentiation between high and low grade tumours in paediatric patients by using apparent diffusion coefficients. Eur J PaediatrNeurol 17:302–307
Kralik SF, Taha A, Kamer AP, Cardinal JS, Seltman TA, Ho CY (2014) Diffusion imaging for tumor grading of supratentorial brain tumors in the first year of life. AJNR Am J Neuroradiol 35:815–823
LaViolette PS, Mickevicius NJ, Cochran EJ et al (2014) Precise ex vivo histological validation of heightened cellularity and diffusion-restricted necrosis in regions of dark apparent diffusion coefficient in 7 cases of high-grade glioma. Neuro-Oncology 16:1599–1606
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignantgliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Ulmer S, Braga TA, Barker FG 2nd, Lev MH, Gonzalez RG, Henson JW (2006) Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 67:1668–1670
Kruser TJ, Mehta MP, Robins HI (2013) Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 13:389–403
Hygino da Cruz LC, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of Posttreatmentglioma. Am J Neuroradiol 32:1978–1985
Reardon DA, Gokhale PC, Klein SR et al (2015) Glioblastomaeradication following immune checkpoint blockade in an orthotopic, Immunocompetentmodel. Cancer Immunol Res 4:124–135
Hilario A, Sepulveda JM, Perez-Nunez A et al (2014) A prognostic model based on preoperative MRI predicts overall survival in patients with diffuse gliomas. AJNR Am J Neuroradiol 35:1096–1102
Prager AJ, Martinez N, Beal K, Omuro A, Zhang Z, Young RJ (2015) Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol 36:877–885
Nagane M, Kobayashi K, Tanaka M et al (2014) Predictive significance of mean apparent diffusion coefficient value for responsiveness of temozolomide-refractory malignant glioma to bevacizumab: preliminary report. Int J ClinOncol 19:16–23
Chen C, Huang R, MacLean A et al (2013) Recurrent high-grade glioma treated with bevacizumab: prognostic value of MGMT methylation, EGFR status and pretreatment MRI in determining response and survival. J Neuro-Oncol 115:267–276
Mong S, Ellingson BM, Nghiemphu PL et al (2012) Persistent diffusion-restricted lesions in bevacizumab-treated malignant gliomas are associated with improved survival compared with matched controls. AJNR Am J Neuroradiol 33:1763–1770
Yamasaki F, Sugiyama K, Ohtaki M et al (2010) Glioblastoma treated with postoperative radio-chemotherapy: prognostic value of apparent diffusion coefficient at MR imaging. Eur J Radiol 73:532–537
Yamasaki F, Kurisu K, Satoh K et al (2005) Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 235:985–991
Watling CJ, Lee DH, Macdonald DR, Cairncross JG (1994) Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J ClinOncol 12:1886–1889
Goh JJ, See SJ, Ang E, Ng WH (2009) Vanishing glioblastoma after corticosteroid therapy. J ClinNeurosci 16:1226–1228
Elson A, Bovi J, Siker M, Schultz C, Paulson E (2015) Evaluation of absolute and normalized apparent diffusion coefficient (ADC) values within the post-operative T2/FLAIR volume as adverse prognostic indicators in glioblastoma. J Neuro-Oncol 122:549–558
Acknowledgments
We thank Annie G. Bryant, Northeastern University, for assistance with the manuscript preparation and submission.
Conflict of interest statement
DAR reports personal fees from AbbVie, personal fees from Bristol Myers Squibb, personal fees from Cavion, grants and personal fees from Celldex, personal fees from Genentech Roche, grants and personal fees from Inovio, personal fees from Juno Pharmaceuticals, personal fees from Merck, grants and personal fees from Midatech, personal fees from Momenta Pharmaceuticals, personal fees from Novartis, personal fees from Novocure, personal fees from Oxigene, personal fees from Regeneron, personal fees from Stemline Therapeutics, and grants from Incyte, outside the submitted work
Author information
Authors and Affiliations
Corresponding authors
Additional information
LQ and XL contributed equally to this work.
Electronic supplementary material
Table S1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Qin, L., Li, X., Stroiney, A. et al. Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma. Neuroradiology 59, 135–145 (2017). https://doi.org/10.1007/s00234-016-1769-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1769-8