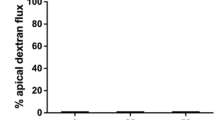
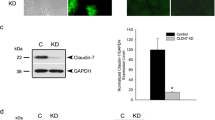
Abstract
Studies have reported that Na,K-ATPase interacts with E-cadherin to stabilize (AJs) and regulate the expression of claudins, the main proteins present in the tight junction (TJ) in epithelial cells containing caveolae. However, the role of this ATPase in the regulation of the AJ and TJ proteins in colorectal cancer cells as well as the molecular events underlying this event in a caveolae-independent system remain undefined. In the present study, we used ouabain, a classic drug known to inhibit Na,K-ATPase, and Caco-2 cells, which are a well-established human colorectal cancer model that does not exhibit caveolae. We demonstrated that ouabain treatment resulted in a reduction of the β1 Na,K-ATPase protein and cell redistribution of the AJ proteins E-cadherin and β-catenin, as well as the α1 Na,K-ATPase subunit. Furthermore, ouabain increased claudin-3 protein levels, impaired the TJ barrier function and increased cell viability and proliferation during the early stages of treatment. Additionally, the observed ouabain-induced events were dependent on the activation of ERK1/2 signaling; but in contrast to previous studies, this signaling cascade was caveolae-independent. In conclusion, our findings strongly suggest that α1 and β1 Na,K-ATPase downregulation and ERK1/2 activation induced by ouabain are interlinked events that play an important role during cell–cell adhesion loss, which is an important step during the tumor progression of colorectal carcinomas.






Similar content being viewed by others
References
Bagrov AY, Shapiro JI, Fedorova OV (2009) Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Am J Hypertens 22(5):559–563
Barbosa LA, Goto-Silva L, Redondo PA et al (2003) TPA-induced signal transduction: a link between PKC and EGFR signaling modulates the assembly of intercellular junctions in Caco-2 cells. Cell Tissue Res 312:319–331
Breuza L, Corby S, Arsanto JP et al (2002) The scaffolding domain of caveolin 2 is responsible for its Golgi localization in Caco-2 cells. J Cell Sci 115(Pt 23):4457–4467
Cereijido M, Contreras RG, Shoshani L, Larre I (2012) The Na+-K+-ATPase as self-adhesion molecule and hormone receptor. Am J Physiol Cell Physiol 302:C473–C481
Contreras RG, Flores-Maldonado C, Lazaro A et al (2004) Ouabain binding to Na+, K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus. J Membr Biol 198:147–158
de Freitas JC Jr, Silva Bdu R, de Souza WF et al (2011) Inhibition of N-linked glycosylation by tunicamycin induces E-cadherin-mediated cell–cell adhesion and inhibits cell proliferation in undifferentiated human colon cancer cells. Cancer Chemother Pharmacol 68(1):227–238
de Rezende Corrêa G, Araujo dos Santos A, Frederico Leite Fontes C, Giestal de Araujo E (2005) Ouabain induces an increase of retinal ganglion cell survival in vitro: the involvement of protein kinase C. Brain Res 1049(1):89–94
Esteves MB, Marques-Santos LF, Affonso-Mitidieri OR, Rumjanek VM (2005) Ouabain exacerbates activation-induced cell death in human peripheral blood lymphocytes. An Acad Bras Cienc 77(2):281–292
Felth J, Rickardson L, Rosén J et al (2009) Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs. J Nat Prod 72:1969–1974
Ikari A, Takiguchi A, Atomi K, Sugatani J (2011) Epidermal growth factor increases clathrin-dependent endocytosis and degradation of claudin-2 protein in MDCK II cells. J Cell Physiol 226:2448–2456
Inge LJ, Rajasekaran SA, Yoshimoto K et al (2008) Evidence for a potential tumor suppressor role for the Na, K-ATPase beta1-subunit. Histol Histopathol 23:459–467
Kometiani P, Liu L, Askari A (2005) Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol 67:929–936
Krug SM, Günzel D, Conrad MP et al (2012) Charge-selective claudin channels. Ann N Y Acad Sci 1257:20–28
Larre I, Lazaro A, Contreras RG et al (2010) Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci USA 107(25):11387–11392
Larre I, Contreras RG, Cereijido M (2011) Ouabain modulates cell contacts as well as functions that depend on cell adhesion. Methods Mol Biol 763:155–168
Li Q, Mattingly RR (2008) Restoration of E-cadherin cell–cell junctions requires both expression of E-cadherin and suppression of ERK MAP kinase activation in Ras-transformed breast epithelial cells. Neoplasia 10:1444–1458
Li Z, Xie Z (2009) The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch 457:635–644
Liang M, Tian J, Liu L et al (2007) Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem 282(14):10585–10593
Liu J, Shapiro JI (2007) Regulation of sodium pump endocytosis by cardiotonic steroids: molecular mechanisms and physiological implications. Pathophysiology 14:171–181
Liu L, Mohammadi K, Aynafshar B et al (2003) Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol 284:C1550–C1560
Liu L, Ivanov AV, Gable ME et al (2011) Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase. Biochemistry 50(40):8664–8673
Mijatovic T, Van Quaquebeke E, Delest B et al (2007) Cardiotonic steroids on the road to anti-cancer therapy. Biochim Biophys Acta 1776:32–57
Mineta K, Yamamoto Y, Yamazaki Y et al (2011) Predicted expansion of the claudin multigene family. FEBS Lett 585(4):606–612
Mitchell LA, Overgaard CE, Ward C et al (2011) Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol 301:L40–L49
Miyoshi J, Takai Y (2008) Structural and functional associations of apical junctions with cytoskeleton. Biochim Biophys Acta 1778:670–691
Mohammadi K, Kometiani P, Xie Z, Askari A (2001) Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem 276(45):42050–42056
Morth JP, Poulsen H, Toustrup-Jensen MS et al (2009) The structure of the Na+, K+-ATPase and mapping of isoform differences and disease-related mutations. Philos Trans R Soc Lond B 364(1514):217–227
Oliveira SS, Oliveira IM, Souza W, Morgado-Díaz JA (2005) Claudins upregulation in human colorectal cancer. FEBS Lett 579:6179–6185
Rajasekaran SA, Ball WJ Jr, Bander NH et al (1999) Reduced expression of β-subunit of Na, K-ATPase in human clear-cell renal cell carcinoma. J Urol 162:574–580
Rajasekaran SA, Palmer LG, Quan K et al (2001) Na, K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell 12:279–295
Rajasekaran SA, Barwe SP, Rajasekaran AK (2005) Multiple functions of Na, K-ATPase in epithelial cells. Semin Nephrol 25:328–334
Rajasekaran SA, Huynh TP, Wolle DG et al (2010) Na, K-ATPase subunits as markers for epithelial–mesenchymal transition in cancer and fibrosis. Mol Cancer Ther 9:1515–1524
Singh AB, Harris RC (2004) Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem 279(5):3543–3552
Takizawa Y, Kishimoto H, Kitazato T et al (2012) Changes in protein and mRNA expression levels of claudin family after mucosal lesion by intestinal ischemia/reperfusion. Int J Pharm 426(1–2):82–89
Tian J, Li X, Liang M et al (2009) Changes in sodium pump expression dictate the effects of ouabain on cell growth. J Biol Chem 284:14921–14929
Vagin O, Sachs G, Tokhtaeva E (2007) The roles of the Na, K-ATPase beta 1 subunit in pump sorting and epithelial integrity. J Bioenerg Biomembr 39:367–372
Wang H, Haas M, Liang M et al (2004) Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem 279(17):17250–17259
Wang Z, Zheng M, Li Z et al (2009) Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res 69:6556–6564
Weber CR (2012) Dynamic properties of the tight junction barrier. Ann N Y Acad Sci 1257:77–84
Xie Z, Askari A (2002) Na+/K+-ATPase as a signal transducer. Eur J Biochem 269(10):2434–2439
Yap AS, Crampton MS, Hardin J (2007) Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol 19:508–514
Acknowledgments
This research was supported by Ministério da Saúde (Brazil), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Grant E26/170.026/2008), Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant 573806/2008-0) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The text was reviewed by American journal experts.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Effect of low dose of ouabain and ionic imbalance on cell distribution of E-cadherin.Monolayers of Caco-2 cells were grown on glass coverslips. Cells were treated with a low concentration of ouabain (100 nM) for 6 h or with a molar excess of NaCl (250 mM) for 2.5 h. After, cells were processed for immunofluorescence analysis of E-cadherin distribution. Bar = 10 μm (TIFF 629 kb)
Fig. S2
Impact of ERK1/2 pathway inhibition on the ouabain-induced effects in β1 Na,K-ATPase levels. Monolayers of Caco-2 cells were grown, pretreated with an inhibitor of the ERK1/2 pathway (PD98059) for 1 h (when indicated) and incubated with ouabain for 6 h. After treatment, total cell lysates were harvested and the expression of β1 Na,K-ATPase was visualized by immunoblotting. Ponceau staining (Ponc.) was used as a loading control. Numbers represent the ratio of the optical density of treated to untreated cells (TIFF 59 kb)
Rights and permissions
About this article
Cite this article
de Souza, W.F., Barbosa, L.A., Liu, L. et al. Ouabain-Induced Alterations of the Apical Junctional Complex Involve α1 and β1 Na,K-ATPase Downregulation and ERK1/2 Activation Independent of Caveolae in Colorectal Cancer Cells. J Membrane Biol 247, 23–33 (2014). https://doi.org/10.1007/s00232-013-9607-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-013-9607-y