Abstract
Objective: the stereospecificity of mefloquine pharmacokinetics in children has been investigated.
Patients:
Twelve children aged 6 to 24 months were treated for uncomplicated falciparum malaria with a single oral dose of 25 mg⋅kg−1 racemic mefloquine in combination with sulfadoxine and pyrimethamine.
Methods:
concentrations of mefloquine enantiomers were determined using a coupled achiral-chiral chromatographic system. Pharmacokinetic parameters were calculated using model-independent analysis.
Results:
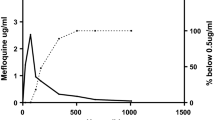
Maximum plasma concentrations, areas under the curve and apparent plasma elimination half-lives were higher for the (−) enantiomer than its antipode. In contrast, the apparent volume of distribution (V/f) and total clearance (Cl/f) values were higher for the (+) enantiomer.
Conclusion:
the stereoselectivity of mefloquine pharmacokinetics is similar to that observed in adults.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 23 March 1995/Accepted in revised form: 26 October 1995
Rights and permissions
About this article
Cite this article
Bourahla, A., Martin, C., Gimenez, F. et al. Stereoselective pharmacokinetics of mefloquine in young children. E J Clin Pharmacol 50, 241–244 (1996). https://doi.org/10.1007/s002280050100
Issue Date:
DOI: https://doi.org/10.1007/s002280050100