Abstract
Aim
To compare survival outcomes, response rates, and adverse events (AEs) in proton pump inhibitor (PPI) user and non-user patients with metastatic colorectal cancer (mCRC) treated with regorafenib.
Methods
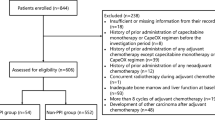
We included 272 patients with mCRC treated with regorafenib in this study. Patients were divided into two categories according to their status of PPI use. The primary endpoint was overall survival (OS). The secondary endpoints were time to treatment failure (TTF), response rates, and safety. To exclude immortal time bias in survival analyses, we compared PPI non-user patients and all patients.
Results
There were 141 and 131 patients in the PPI non-user and user groups. Baseline characteristics were similar in each group. Pantoprazole was the most used PPI. At the median 35.2 (95% confidence interval (CI): 32.6–37.9) months follow-up, the median OS was similar in PPI non-user and all patients (6.9 months (95% CI: 5.3–8.5) and 7.7 months (95% CI:6.6–8.8), p = 0.913). TTF was also similar in PPI non-user and all patients (3.3 months (95% CI: 2.7–3.9) and 3.5 months (95% CI: 3.0–4.0), p = 0.661). In multivariable analysis, no statistically significant difference was observed between PPI user and non-user groups in OS and TTF (hazard ratio (HR), 0.99; 95% CI, 0.77–1.28; p = 0.963 for OS; HR, 0.93; 0.77–1.20, p = 0.598 for TTF). The objective response rates (ORR) were similar in the PPI non-user and user groups (19.8% and 16.8%, p = 0.455). The rates of any grade AEs were also similar in each group.
Conclusion
This study found no worse outcome in the combined use of PPI and regorafenib among patients with mCRC.


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- AEs:
-
Adverse events
- ATP:
-
Adenosine triphosphate
- CI:
-
Confidence interval
- CYP:
-
Cytochrome P450
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- DDI:
-
Drug-drug interactions
- ECOG:
-
Eastern Cooperative Oncology Group
- GIST:
-
Gastrointestinal stromal tumor
- HCC:
-
Hepatocellular cancer
- IQR:
-
Interquartile range
- mCRC:
-
Metastatic colorectal cancer
- ORR:
-
Objective response rates
- OS:
-
Overall survival
- PPI(s):
-
Proton pump inhibitor(s)
- RECIST:
-
Response evaluation criteria in solid tumors
- TKIs:
-
Tyrosine kinase inhibitors
- TTF:
-
Time to treatment failure
References
Kudo M (2020) Recent advances in systemic therapy for hepatocellular carcinoma in an aging society: 2020 Update. Liver Cancer 9(6):640–662. https://doi.org/10.1159/000511001
Crona DJ, Keisler MD, Walko CM (2013) Regorafenib: a novel multitargeted tyrosine kinase inhibitor for colorectal cancer and gastrointestinal stromal tumors. Ann Pharmacother 47(12):1685–1696. https://doi.org/10.1177/1060028013509792
Shirley M, Keating GM (2015) Regorafenib: a review of its use in patients with advanced gastrointestinal stromal tumours. Drugs 75(9):1009–1017. https://doi.org/10.1007/s40265-015-0406-x
Gay C, Toulet D, Le Corre P (2017) Pharmacokinetic drug-drug interactions of tyrosine kinase inhibitors: a focus on cytochrome P450, transporters, and acid suppression therapy. Hematol Oncol 35(3):259–280. https://doi.org/10.1002/hon.2335
Yin OQ, Gallagher N, Fischer D, Demirhan E, Zhou W, Golor G, Schran H (2010) Effect of the proton pump inhibitor esomeprazole on the oral absorption and pharmacokinetics of nilotinib. J Clin Pharmacol 50(8):960–967. https://doi.org/10.1177/0091270009346061
van Leeuwen RWF, Jansman FGA, Hunfeld NG, Peric R, Reyners AKL, Imholz ALT, Brouwers J, Aerts JG, van Gelder T, Mathijssen RHJ (2017) Tyrosine kinase inhibitors and proton pump inhibitors: an evaluation of treatment options. Clin Pharmacokinet 56(7):683–688. https://doi.org/10.1007/s40262-016-0503-3
Triadafilopoulos G, Roorda AK, Akiyama J (2013) Indications and safety of proton pump inhibitor drug use in patients with cancer. Expert Opin Drug Saf 12(5):659–672. https://doi.org/10.1517/14740338.2013.797961
Numico G, Fusco V, Franco P, Roila F (2017) Proton pump inhibitors in cancer patients: how useful they are? A review of the most common indications for their use. Crit Rev Oncol Hematol 111:144–151. https://doi.org/10.1016/j.critrevonc.2017.01.014
Herbrink M, Nuijen B, Schellens JH, Beijnen JH (2015) Variability in bioavailability of small molecular tyrosine kinase inhibitors. Cancer Treat Rev 41(5):412–422. https://doi.org/10.1016/j.ctrv.2015.03.005
Ohgami M, Kaburagi T, Kurosawa A, Doki K, Shiozawa T, Hizawa N, Homma M (2018) Effects of proton pump inhibitor coadministration on the plasma concentration of erlotinib in patients with non-small cell lung cancer. Ther Drug Monit 40(6):699–704. https://doi.org/10.1097/FTD.0000000000000552
van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG (2014) Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol 15(8):e315-326. https://doi.org/10.1016/S1470-2045(13)70579-5
McAlister RK, Aston J, Pollack M, Du L, Koyama T, Chism DD (2018) Effect of Concomitant pH-elevating medications with pazopanib on progression-free survival and overall survival in patients with metastatic renal cell carcinoma. Oncologist 23(6):686–692. https://doi.org/10.1634/theoncologist.2017-0578
Wu CY, Ho HJ, Wu CY, Chen YJ, Lee TY, Hsu YC, Lin JT (2020) Association between proton pump inhibitor use and mortality in patients with hepatocellular carcinoma receiving tyrosine kinase inhibitor. Gut (Online ahead of print, 2020/09/11). https://doi.org/10.1136/gutjnl-2020-321932
de Man FM, Hussaarts K, de With M, Oomen-de Hoop E, de Bruijn P, van Halteren HK, van der Burg-de GN, Eskens F, van Gelder T, van Leeuwen RWF, Mathijssen RHJ (2019) Influence of the proton pump inhibitor esomeprazole on the bioavailability of regorafenib: a randomized crossover pharmacokinetic study. Clin Pharmacol Ther 105(6):1456–1461. https://doi.org/10.1002/cpt.1331
Wisinski KB, Cantu CA, Eickhoff J, Osterby K, Tevaarwerk AJ, Heideman J, Liu G, Wilding G, Johnston S, Kolesar JM (2015) Potential cytochrome P-450 drug-drug interactions in adults with metastatic solid tumors and effect on eligibility for Phase I clinical trials. Am J Health Syst Pharm 72(11):958–965. https://doi.org/10.2146/ajhp140591
(2013) Summary of Product Characteristics. In: Regorafenib, INN, 1 ed. European Medicines Agency
El Rouby N, Lima JJ, Johnson JA (2018) Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol 14(4):447–460. https://doi.org/10.1080/17425255.2018.1461835
Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF (2001) Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedebergs Arch Pharmacol 364(6):551–557. https://doi.org/10.1007/s00210-001-0489-7
Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone F, Federici C, Iessi E, Parmiani G, Arancia G, Belardelli F, Fais S (2004) Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst 96(22):1702–1713. https://doi.org/10.1093/jnci/djh305
Fujita KI, Masuo Y, Yamazaki E, Shibutani T, Kubota Y, Nakamichi N, Sasaki Y, Kato Y (2017) Involvement of the transporters p-glycoprotein and breast cancer resistance protein in dermal distribution of the multikinase inhibitor regorafenib and its active metabolites. J Pharm Sci 106(9):2632–2641. https://doi.org/10.1016/j.xphs.2017.04.064
Koo MM, von Wagner C, Abel GA, McPhail S, Hamilton W, Rubin GP, Lyratzopoulos G (2018) The nature and frequency of abdominal symptoms in cancer patients and their associations with time to help-seeking: evidence from a national audit of cancer diagnosis. J Public Health (Oxf) 40(3):e388–e395. https://doi.org/10.1093/pubmed/fdx188
Wu CY, Ho HJ, Wu CY, Chen YJ, Lee TY, Hsu YC, Lin JT (2020) Association between proton pump inhibitor use and mortality in patients with hepatocellular carcinoma receiving tyrosine kinase inhibitor. Gut. https://doi.org/10.1136/gutjnl-2020-321932
Raoul JL, Edeline J, Simmet V, Moreau-Bachelard C, Gilabert M, Frenel JS (2022) Long-term use of proton pump inhibitors in cancer patients: an opinion paper. Cancers (Basel) 14 (5). https://doi.org/10.3390/cancers14051156
Shimura S, Hamamoto N, Yoshino N, Kushiyama Y, Fujishiro H, Komazawa Y, Furuta K, Ishihara S, Adachi K, Kinoshita Y (2012) Diarrhea caused by proton pump inhibitor administration: comparisons among lansoprazole, rabeprazole, and omeprazole. Curr Ther Res Clin Exp 73(3):112–120. https://doi.org/10.1016/j.curtheres.2012.03.002
Law EH, Badowski M, Hung YT, Weems K, Sanchez A, Lee TA (2017) Association Between proton pump inhibitors and microscopic colitis. Ann Pharmacother 51(3):253–263. https://doi.org/10.1177/1060028016673859
Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, Chiriac SA, Ciobica A, Boiculese L (2017) Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol 23(35):6500–6515. https://doi.org/10.3748/wjg.v23.i35.6500
Xie Y, Bowe B, Yan Y (2019) Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 365:I1580. https://doi.org/10.1136/bmj.l1580
Author information
Authors and Affiliations
Contributions
Emre Yekedüz: Conceptualization, data curation, methodology, visualization, formal analysis, writing–original draft. Mehmet Fatih Özbay: Data curation, writing–review and editing. Dilek Çağlayan: Data curation, writing–review and editing. Atila Yıldırım: Data curation, writing–review and editing. Cihan Erol: Data curation, writing–review and editing. Hasan Çağrı Yıldırım: Data curation, writing–review and editing. Sezai Tunç: Data curation, writing–review and editing. Neslihan Özyurt: Data curation, writing–review and editing. Feyyaz Özdemir: Writing–review and editing, Supervision. Mehmet Ali Nahit Şendur: Writing, review and editing; supervision. Abdurrahman Işıkdoğan: Writing, review and editing; supervision. Saadettin Kılıçkap: Writing, review and editing; supervision. Yüksel Ürün: Methodology, Writing, review and editing; supervision. Şuayib Yalçın: Writing, review and editing; supervision. Mehmet Artaç: Writing, review and editing; supervision. Hasan Şenol Coşkun: Writing, review and editing; supervision. Güngör Utkan: Conceptualization, methodology, data curation, writing (review and editing), supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Ankara University Faculty of Medicine Human Research Ethics Committee, approval number İ9-601-21.
Consent to participate
Waiver of informed consent was obtained from the Research Ethics Board due to the minimal risk to study subjects as well as the retrospective nature of this study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yekedüz, E., Özbay, M.F., Çağlayan, D. et al. Clinical outcomes of concomitant use of proton pump inhibitors and regorafenib in patients with metastatic colorectal cancer: a multicenter study. Eur J Clin Pharmacol 78, 1973–1979 (2022). https://doi.org/10.1007/s00228-022-03403-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03403-1