Abstract
Purpose
To estimate the prevalence of mortality among patients that develop adverse drug reactions during hospitalisation (ADRIn), to examine heterogeneity through subgroup analysis and to identify system-organ class (SOC) and their causative drugs.
Methods
Two investigators searched PubMed, Google Scholar and related bibliography for studies reporting ADRIn-related mortality data. The primary outcome was to compute overall prevalence of fatal ADRIn (95% CI) using double arcsine method. We explored the heterogeneity (I2) in its estimation based on study design, study population and data collection methods. The secondary outcomes were the pattern of fatal reactions and their causative drugs. PROSPERO register number—CRD42018090331.
Results
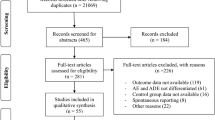
Out of 349 full text assessed, 48 studies satisfying the selection criteria were included. The fatal ADRIn prevalence was 0.11% (95% CI 0.06–0.18%; I2 = 93%). The fatal ADRIn prevalence ranged from 0.03% (I2 = 0%) in all ages to 0.27% (I2 = 90%) in elderly population studies. Elderly studies varied for all study characteristics. Among study wards, a higher trend of prevalence was observed in ‘internal medicine and ICU’ (0.46%, I2 = 51%) and ‘neonatal/paediatric ward and ICU’ (0.34%, I2 = 58%) studies. The commonly involved SOC were ‘gastrointestinal disorders’ (28.79%), ‘blood and lymphatic system disorders’ (19.69%) and ‘renal and urinary disorders’ (13.64%). Most commonly observed causative drug-fatal ADRIn pairs were antithrombotics and nonsteroidal anti-inflammatory drugs induced gastrointestinal bleeding, and antineoplastic agents induced cytopenia.
Conclusion
ADRIn is an important cause of mortality. Age groups and study wards have important influence on prevalence of fatal ADRIn and its heterogeneity across studies. Few class drugs contribute to sizable proportion of ADRIn-related mortality.


Similar content being viewed by others
References
Oscanoa TJ, Lizaraso F, Carvajal A (2017) Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol 73(6):759–770
Bouvy JC, De Bruin ML, Koopmanschap MA (2015) Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf 38(5):437–453
Beijer HJM, De Blaey CJ (2002) Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 24(2):46–54
Kongkaew C, Noyce PR, Ashcroft DM (2008) Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 42(7):1017–1025
Lazarou J, Pomeranz BH, Corey PN (1998) Prevalence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279(15):1200–1205
Patel TK, Patel PB (2018) Mortality among patients due to adverse drug reactions that lead to hospitalization: a meta-analysis. Eur J Clin Pharmacol 74(6):819–832
Patel NS, Patel TK, Patel PB, Naik VN, Tripathi CB (2017) Hospitalizations due to preventable adverse reactions-a systematic review. Eur J Clin Pharmacol 73(4):385–398
Angamo MT, Chalmers L, Curtain CM, Bereznicki LR (2016) Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf 39(9):847–857
Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M (2009) Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One 4(2):e4439
Suh DC, Woodall BS, Shin SK, Hermes-De Santis ER (2000) Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother 34(12):1373–1379
Miguel A, Azevedo LF, Araújo M, Pereira AC (2012) Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 21(11):1139–1154
Patel TK, Patel PB (2016) Incidence of adverse drug reactions in Indian hospitals: a systematic review of prospective studies. Curr Drug Saf 11(2):128–136
Pardo Cabello AJ, Del Pozo Gavilán E, Gómez Jiménez FJ, Mota Rodríguez C, Luna Del Castillo Jde D, Puche Cañas E (2016) Drug-related mortality among inpatients: a retrospective observational study. Eur J Clin Pharmacol 72(6):731–736
Smyth RMD, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R et al (2012) Adverse drug reactions in children—a systematic review. PLoS One 7(3):e24061
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T (2013) Meta-analysis of prevalence. J Epidemiol Community Health 67(11):974–978
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Aljadhey H, Mahmoud MA, Mayet A, Alshaikh M, Ahmed Y, Murray MD et al (2013) Incidence of adverse drug events in an academic hospital: a prospective cohort study. Int J Qual Health Care 25(6):648–655
Aljadhey H, Mahmoud MA, Ahmed Y, Sultana R, Zouein S, Alshanawani S et al (2016) Incidence of adverse drug events in public and private hospitals in Riyadh, Saudi Arabia: the (ADESA) prospective cohort study. BMJ Open 6(7):e010831
Alsbou M (2010) Incidence of adverse drug reactions in Alkarak hospital: a pilot study. J Med J 44(4):442–446
Andrade PHS, Lobo IMF, da Silva WB (2017) Risk factors for adverse drug reactions in pediatric inpatients: a cohort study. PLoS One 12(8):e0182327
Belén Rivas A, Arruza L, Pacheco E, Portoles A, Diz J, Vargas E (2016) Adverse drug reactions in neonates: a prospective study. Arch Dis Child 101(4):371–376
Benkirane RR, Abouqal R, Haimeur CC, Ech Cherif S, El Kettani SS, Azzouzi AA, Mdaghri Alaoui AA et al (2009) Incidence of adverse drug events and medication errors in intensive care units: a prospective multicenter study. J Patient Saf 5(1):16–22
Calderón-Ospina C, Bustamante-Rojas C (2010) The DoTS classification is a useful way to classify adverse drug reactions: a preliminary study in hospitalized patients. Int J Pharm Pract 18(4):230–235
Davies EC, Green CF, Mottram DR, Pirmohamed M (2006) Adverse drug reactions in hospital in-patients: a pilot study. J Clin Pharm Ther 31(4):335–341
de Las Salas R, Díaz-Agudelo D, Burgos-Flórez FJ, Vaca C, Serrano-Meriño DV (2016) Adverse drug reactions in hospitalized Colombian children. Colomb Med (Cali) 47(3):142–147
de Oliveira-Filho AD, Vieira AES, da Silva RC, Neves SJF, Gama TAB, Lima RV et al (2017) Adverse drug reactions in high-risk pregnant women: a prospective study. Saudi Pharm J 25(7):1073–1077
Dedefo MG, Mitike AH, Angamo MT (2016) Incidence and determinants of medication errors and adverse drug events among hospitalized children in West Ethiopia. BMC Pediatr 16:81
Doshi MS, Patel PP, Shah SP, Dikshit RK (2012) Intensive monitoring of adverse drug reactions in hospitalized patients of two medical units at a tertiary care teaching hospital. J Pharmacol Pharmacother 3(4):308–313
El Sebaee HA, El Salam Seloma YA (2013) Adverse drug reactions among critically ill patients at Cairo University Hospital: frequency and outcomes. J Biol Agr Healthc 3(13):5–13
Fattahi F, Pourpak Z, Moin M, Kazemnejad A, Khotaei GT, Mamishi S et al (2005) Adverse drug reactions in hospitalized children in a department of infectious diseases. J Clin Pharmacol 45(11):1313–1318
Fattinger K, Roos M, Vergères P, Holenstein C, Kind B, Masche U et al (2000) Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 49(2):158–167
Ganeva M, Gancheva T, Troeva J, Gancheva D, Hristakieva E (2016) A study of adverse drug reactions in hospitalized patients in relation to age. Eur J Clin Pharm 18(3):154–162
Geer MI, Koul PA, Tanki SA, Shah MY (2016) Frequency, types, severity, preventability and costs of adverse drug reactions at a tertiary care hospital. J Pharmacol Toxicol Methods 81:323–334
Gor AP, Desai SV (2008) Adverse drug reactions (ADR) in the in patients of Medicine Department of a Rural Tertiary Care Teaching Hospital and influence of pharmacovigilance in reporting ADR. Indian J Pharmacol 40(1):37–40
Haffner S, von Laue N, Wirth S, Thürmann PA (2005) Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf 28(5):453–464
Haile DB, Ayen WY, Tiwari P (2013) Prevalence and assessment of factors contributing to adverse drug reactions in wards of a tertiary care hospital, India. Ethiop J Health Sci 23(1):39–48
Härkänen M, Kervinen M, Ahonen J, Voutilainen A, Turunen H, Vehviläinen-Julkunen K (2015) Patient-specific risk factors of adverse drug events in adult inpatients - evidence detected using the global trigger tool method. J Clin Nurs 24(3–4):582–591
Harugeri A, Parthasarathi G, Ramesh M, Guido S, Basavanagowdappa H (2011) Frequency and nature of adverse drug reactions in elderly in-patients of two Indian medical college hospitals. J Postgrad Med 57(3):189–195
Kathiria JM, Sattigeri BM, Desai PM, Patel SP (2013) A study of adverse drug reactions in patients admitted to intensive care unit of a tertiary care teaching rural hospital. Int J Pharm Pharm Sci 5(1):160–163
Kaur S, Kapoor V, Mahajan R, Lal M, Gupta S (2011) Monitoring of incidence, severity, and causality of adverse drug reactions in hospitalized patients with cardiovascular disease. Indian J Pharmacol 43(1):22–26
Kiguba R, Karamagi C, Bird SM (2017) Incidence, risk factors and risk prediction of hospital-acquired suspected adverse drug reactions: a prospective cohort of Ugandan inpatients. BMJ Open 7(1):e010568
Klopotowska JE, Wierenga PC, Stuijt CC, Arisz L, Dijkgraaf MG, Kuks PF et al (2013) Adverse drug events in older hospitalized patients: results and reliability of a comprehensive and structured identification strategy. PLoS One 8(8):e71045
Kourorian Z, Fattahi F, Pourpak Z, Rasoolinejad M, Gholami K (2009) Adverse drug reactions in an Iranian department of adult infectious diseases. East Mediterr Health J 15(6):1351–1357
Lapatto-Reiniluoto O, Patinen L, Niemi M, Backman JT, Neuvonen PJ (2015) Drug-related inadvertent deaths in a university hospital--a declining trend. Basic Clin Pharmacol Toxicol 117(6):421–426
Mandha M, Reddy KP, Reddy KP (2013) Evaluation of adverse drug reactions in pediatric patients. Indian J Pharm P 6(3):32–35
Mehta U, Durrheim DN, Blockman M, Kredo T, Gounden R, Barnes KI (2008) Adverse drug reactions in adult medical inpatients in a South African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol 65(3):396–406
Montané E, Arellano AL, Sanz Y, Roca J, Farré M (2018) Drug-related deaths in hospital inpatients: a retrospective cohort study. Br J Clin Pharmacol 84(3):542–552
Nebeker JR, Hoffman JM, Weir CR, Bennett CL, Hurdle JF (2005) High rates of adverse drug events in a highly computerized hospital. Arch Intern Med 165(10):1111–1116
Nguyen JK, Fouts MM, Kotabe SE, Lo E (2006) Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother 4(1):36–41
Oshikoya KA, Chukwura H, Njokanma OF, Senbanjo IO, Ojo I (2011) Incidence and cost estimate of treating pediatric adverse drug reactions in Lagos, Nigeria. Sao Paulo Med J 129(3):153–164
Park S, In Y, Suh GY, Sohn K, Kim E (2013) Evaluation of adverse drug reactions in medical intensive care units. Eur J Clin Pharmacol 69(1):119–131
Patel N, Desai S (2015) Profile of adverse drug reactions in patients admitted to general surgical wards of a rural tertiary-care hospital in India. Drugs Ther Perspect 31:402–406
Peter JV, Varghese GH, Alexander H, Tom NR, Swethalekshmi V, Truman C et al (2016) Patterns of adverse drug reaction in the medical wards of a teaching hospital: a prospective observational cohort study. Curr Drug Saf 11(2):164–171
Rothschild JM, Landrigan CP, Cronin JW, Kaushal R, Lockley SW, Burdick E et al (2005) The critical care safety study: the incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med 33(8):1694–1700
Sivasankaran P, Gupta M, Satyanarayan RB, Durai R (2016) Pattern of adverse drug reactions in a govt. district headquarters hospital in Tamilnadu, India. Indian J Pharm P 9(1):33–36
Tangiisuran B, Davies JG, Wright JE, Rajkumar C (2012) Adverse drug reactions in a population of hospitalized very elderly patients. Drugs Aging 29(8):669–679
Thiesen S, Conroy EJ, Bellis JR, Bracken LE, Mannix HL, Bird KA et al (2013) Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children - a prospective observational cohort study of 6,601 admissions. BMC Med 11:237
Thuermann PA, Windecker R, Steffen J, Schaefer M, Tenter U, Reese E et al (2002) Detection of adverse drug reactions in a neurological department: comparison between intensified surveillance and a computer-assisted approach. Drug Saf 25(10):713–724
Tumwikirize WA, Ogwal-Okeng JW, Vernby A, Anokbonggo WW, Gustafsson LL, Lundborg SC (2011) Adverse drug reactions in patients admitted on internal medicine wards in a district and regional hospital in Uganda. Afr Health Sci 11(1):72–78
Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H (2008) Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals. Drug Saf 31(9):789–798
Zoppi M, Braunschweig S, Kuenzi UP, Maibach R, Hoigné R (2000) Incidence of lethal adverse drug reactions in the comprehensive hospital drug monitoring, a 20-year survey, 1974-1993, based on the data of Berne/St. Gallen. Eur J Clin Pharmacol 56(5):427–430
Mouton JP, Mehta U, Parrish AG, Wilson DP, Stewart A, Njuguna CW et al (2015) Mortality from adverse drug reactions in adult medical inpatients at four hospitals in South Africa: a cross-sectional survey. Br J Clin Pharmacol 80(4):818–826
Generali J (2015) Critical care series. Hosp Pharm 50(1):5–6
Devi P, Kamath DY, Anthony N, Santosh S, Dias B (2012) Patterns, predictors and preventability of adverse drug reactions in the coronary care unit of a tertiary care hospital. Eur J Clin Pharmacol 68(4):427–433
Alhawassi TM, Krass I, Bajorek BV, Pont LG (2014) A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging 9:2079–2086
Al Hamid A, Ghaleb M, Aljadhey H, Aslanpour Z (2014) A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br J Clin Pharmacol 78(2):202–217
Laatikainen O, Miettunen J, Sneck S, Lehtiniemi H, Tenhunen O, Turpeinen M (2017) The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur J Clin Pharmacol 73(12):1539–1549
Shoeb M, Fang MC (2013) Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis 35(3):312–319
Baglin T, Barrowcliffe TW, Cohen A, Greaves M, British Committee for Standards in Haematology (2006) Guidelines on the use and monitoring of heparin. Br J Haematol 133(1):19–34
Makris M, Van Veen JJ, Tait CR, Mumford AD, Laffan M, British Committee for Standards in Haematology (2013) Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol 160(1):35–46
Straube S, Tramèr MR, Moore RA, Derry S, McQuay HJ (2009) Mortality with upper gastrointestinal bleeding and perforation: effects of time and NSAID use. BMC Gastroenterol 9:41
Sostres C, Gargallo CJ, Lanas A (2013) Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther 15(Suppl 3):S3
Macie C, Forbes L, Foster GA, Douketis JD (2004) Dosing practices and risk factors for bleeding in patients receiving enoxaparin for the treatment of an acute coronary syndrome. Chest 125(5):1616–1621
de Abajo FJ, Gil MJ, Bryant V, Timoner J, Oliva B, García-Rodríguez LA (2013) Upper gastrointestinal bleeding associated with NSAIDs, other drugs and interactions: a nested case-control study in a new general practice database. Eur J Clin Pharmacol 69(3):691–701
Schjerning Olsen AM, Gislason GH, McGettigan P, Fosbøl E, Sørensen R, Hansen ML et al (2015) Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA 313(8):805–814
Lamberts M, Lip GY, Hansen ML, Lindhardsen J, Olesen JB, Raunsø J et al (2014) Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med 161(10):690–698
Shalman D, Gerson LB (2015) Systematic review with meta-analysis: the risk of gastrointestinal haemorrhage post-polypectomy in patients receiving anti-platelet, anti-coagulant and/or thienopyridine medications. Aliment Pharmacol Ther 42(8):949–956
Lalami Y, Klastersky J (2017) Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: an overview about well-established and recently emerging clinical data. Crit Rev Oncol Hematol 120:163–179
Shitara K, Matsuo K, Oze I, Mizota A, Kondo C, Nomura M et al (2011) Meta-analysis of neutropenia or leukopenia as a prognostic factor in patients with malignant disease undergoing chemotherapy. Cancer Chemother Pharmacol 68(2):301–307
Tan X, Wen Q, Wang R, Chen Z (2017) Chemotherapy-induced neutropenia and the prognosis of colorectal cancer: a meta-analysis of cohort studies. Expert Rev Anticancer Ther 17(11):1077–1085
Slimings C, Riley TV (2014) Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 69(4):881–891
Wilcox MH, Chalmers JD, Nord CE, Freeman J, Bouza E (2017) Role of cephalosporins in the era of Clostridium difficile infection. J Antimicrob Chemother 72(1):1–18
Spigaglia P (2016) Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis 3(1):23–42
Patton A, Davey P, Harbarth S, Nathwani D, Sneddon J, Marwick CA (2018) Impact of antimicrobial stewardship interventions on Clostridium difficile infection and clinical outcomes: segmented regression analyses. J Antimicrob Chemother 73(2):517–526
Wu X, Zhang W, Ren H, Chen X, Xie J, Chen N (2014) Diuretics associated acute kidney injury: clinical and pathological analysis. Ren Fail 36(7):1051–1055
Bagshaw SM, Delaney A, Haase M, Ghali WA, Bellomo R (2007) Loop diuretics in the management of acute renal failure: a systematic review and meta-analysis. Crit Care Resusc 9(1):60–68
Davis A, Gooch I (2006) Best evidence topic report. The use of loop diuretics in acute renal failure in critically ill patients to reduce mortality, maintain renal function, or avoid the requirements for renal support. Emerg Med J 23(7):569–570
Nadeau-Fredette AC, Bouchard J (2013) Fluid management and use of diuretics in acute kidney injury. Adv Chronic Kidney Dis 20(1):45–55
Selby NM, Shaw S, Woodier N, Fluck RJ, Kolhe NV (2009) Gentamicin-associated acute kidney injury. QJM 102(12):873–880
Srisung W, Teerakanok J, Tantrachoti P, Karukote A, Nugent K (2017) Surgical prophylaxis with gentamicin and acute kidney injury: a systematic review and meta-analysis. Ann Transl Med 5(5):100
Nayak-Rao S (2010) Aminoglycoside use in renal failure. Indian J Nephrol 20(3):121–124
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
Risk of bias summary (JPG 108 kb)
Supplementary Figure 2
Meta-analytic summary of subgroup analysis of prevalence of fatal ADRIn according to age groups through random effect model (JPG 679 kb)
Supplementary Figure 3
Meta-analytic summary of subgroup analysis of prevalence of fatal ADRIn according to study wards through random effect model (JPG 814 kb)
Supplementary Table 1
(DOCX 37 kb)
Supplementary Table 2
(DOCX 16 kb)
Supplementary Table 3
(DOCX 13.2 kb)
Supplementary file 1
(DOCX 24.8 kb)
Rights and permissions
About this article
Cite this article
Patel, P.B., Patel, T.K. Mortality among patients due to adverse drug reactions that occur following hospitalisation: a meta-analysis. Eur J Clin Pharmacol 75, 1293–1307 (2019). https://doi.org/10.1007/s00228-019-02702-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02702-4