Abstract
Purpose
Potentially inappropriate medication (PIM) use causes preventable adverse drug reactions in older patients. Several assessment tools have been published to identify and avoid PIM use. In this systematic literature review, we aim to provide summaries and comparisons of validated PIMs lists published between 1991 and 2017 internationally.
Methods
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA), we performed a systematic review of articles describing the development and validation of criteria for identification of PIMs among older people published between January 1991 and April 2017. The searches were conducted on PUBMED, AgeLine, Academic Search, Academic Search Premier, and CINAHL. We identified the most common medications/classes described as PIM. We also identified the drug–disease interactions and drug–drug interactions reported among criteria.
Results
From 2933 articles screened, 36 met our inclusion criteria. The majority used the Delphi method to validate their criteria. We identified 907 different medications/classes, 536 different drug disease interactions involving 84 diseases/conditions, and 159 drug–drug interactions. Benzodiazepines and nonsteroidal anti-inflammatory drugs were the medications most commonly reported as potentially inappropriate for older people.
Conclusion
Although approaches aimed at detecting inappropriate prescribing have intensified in recent years, we observed limited overlap between different PIM lists. Additionally, some PIM lists did not provide special considerations of use and alternative therapies to avoid PIMs. These facts may compromise the use of PIM lists in clinical practice. Future PIM lists should integrate information about alternative therapies and special considerations of use in order to help clinicians in the drug prescription.
Similar content being viewed by others
Introduction
As the complexity of pharmacotherapy has increased with increasing medication use, particularly among older adults with multiple morbidities [1], medication risk management has become an increasingly important area of research. In this field, potentially inappropriate medication (PIM) is a term used to describe the use of a medicine for which the associated risks outweigh the potential benefits, especially when more effective alternatives are available [2]. PIM use is an important public health challenge, with high prevalence rates (from 18 to > 40%) across a variety of healthcare settings [3,4,5,6]. Notably, elderly patients are more likely to be exposed to PIMs because they often deal with age-related pharmacokinetic and pharmacodynamic changes, which can result in increased adverse drug reactions and decreased efficacy [7, 8]. Additionally, older patients often suffer from multiple chronic-degenerative diseases and therefore use a higher number of drugs, compared to other age groups [9]. In this population, PIM use can lead to avoidable adverse drug events (ADEs) [3, 10], including falls, fractures, and delirium and is associated with hospitalization [11,12,13] and mortality [14,15,16].
In recent years, many strategies and tools have been developed to assess the appropriateness of medication use in older people [2, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Explicit criteria (EC) focusing on a single medication/medication class can support improvements to the quality of drug therapy and help to monitor drug therapy. However, the development of evidence-based PIM lists specifically for older populations is problematic, as older people are typically underrepresented or excluded from most efficacy and safety trials [52, 53]. Accordingly, some investigators have used a consensus technique that synthesizes accumulated expert opinion to develop EC that would facilitate the formulation of recommendations for suitable treatments in older people [54]. This consensus technique could be used to determine which statements from the literature are applicable in clinical practice [22].
Many different expert panels, including pharmacists, geriatricians, and other health professionals, have developed lists of EC to identify PIM use among older people in different countries [2, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Previous systematic reviews identified 7–25 different PIM lists applied to older people [55,56,57]. Although Luchetti et al. [57] summarized and described all medications classified as PIMs using 14 validated and nonvalidated PIM lists published between 2006 and 2015, the literature lacks a comprehensive evaluation of the most common drug–disease and drug–drug interactions described in these validated PIM lists. Notably, summaries of the items proven valid by many consensus panels may facilitate a translational comparison of the processes and provide information about the most important PIMs in clinical practice, which would inform the development of interventions aimed at improving the prescription of specific medications. Therefore, in this review, we aim to summarize and compare the validated potentially inappropriate medications lists for older people published in different countries between 1991 and 2017. Additionally, we aim to summarize the medications and drug–disease and drug–drug interactions listed in the different potentially inappropriate medications lists.
Methods
This review was performed according to a standard protocol for systematic reviews, which was based on the methodological manuals of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The PRISMA checklist is available in Appendix 1.
Search strategy
We systematically identified studies published between January 1991 and April 2017 without any language restriction in the PUBMED and Academic Search Complete via EBSCOhost, Academic Search Premier via EBSCOhost, AgeLine via EBSCOhost, and CINAHL via EBSCOhost electronic databases. We selected this time limit for publication inclusion because the first instrument for PIM assessment was published in 1991 by Beers et al. [22]. The search included terms related to older adults or appropriate/inappropriate medication. Details of the full search strategy are included in Appendix 2.
Eligibility criteria
Original studies describing the EC used to determine potentially inappropriate medications were considered eligible for inclusion in this review if they involved individuals aged 65 years and older and described the development and validation of the methods used in the PIM list. Interventions and observational studies that evaluated PIMs were also retained if the abstract described potentially relevant PIM lists.
We applied the following exclusion criteria: medication review techniques using implicit criteria to evaluate PIMs and lists of PIMs restricted to specific therapeutic classes or specific diseases. Additionally, we excluded studies of PIMs not validated by expert consensus and guidelines or recommendations for the assessment of inappropriate prescriptions, as well as letters, editorials, and duplicate studies.
Study selection
Duplicate manuscripts were removed after exporting the search results to Endnote, version X6 (Clarivate Analytics, Philadelphia, PA, USA). Subsequently, two reviewers independently screened the titles and abstracts of the remaining manuscripts to identify potentially relevant studies describing the development and validation of PIM lists. Additional studies were identified by a manual search of the citation lists for studies that detailed potentially relevant PIM lists. Finally, full-text copies of studies that described either the validation or use of any of the potentially relevant measures were retrieved and considered for inclusion in this review. If a decision could not be reached regarding the ability of a manuscript to meet the inclusion criteria, a decision was reached during the following selection round.
Data extraction and synthesis
Two authors (FRM and JSF) independently extracted the data, after which the first author checked the completeness by reviewing the extraction tables generated by the second author and checking the extracted data in the full-text articles. Disagreements were resolved by discussion between the two authors; if no agreement could be reached, a third author was consulted (VMV).
The following data were extracted from the selected articles: country of origin, source of data used, and validation method (consensus technique, expert panel, literature based). We also extracted aspects evaluated in the lists of PIMs (medications, dosage, duration of therapy, duplication, drug–disease interactions, drug–drug interactions). We also analyzed the medication/medication class names and drug–disease interactions (medication or medication dosage or medication duration with consideration of diagnosis) and drug–drug interactions reported in all PIM lists. We considered all medications belonging to a class as inappropriate if the authors described concerns about the medication class and did not describe single medications. However, the anticholinergic medication class exhibited considerable variation in terms of the selection of specific drugs. Therefore, we included anticholinergic drugs described in a recent review of the literature for the EC that did not specifically state which medicines were considered anticholinergic [58]. Additionally, we also considered a medication class as inappropriate when the authors described single medications and raised concerns related to the medication class.
The data were entered into Excel (Microsoft Corp., Redmond, WA, USA), and all individual medications reported in the studies were subsequently grouped into Anatomical, Therapeutic and Chemical (ATC) classes (five levels).
Results
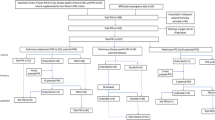
The search strategy produced 2933 potentially relevant publications (Fig. 1). After screening titles and abstracts, we retained 248 potentially relevant publications according to the inclusion criteria. After a full-text review, 214 articles were excluded according to the exclusion criteria. A manual search from the reference lists of the included articles produced two relevant publications not found in the previous systematic database search. Thus, 36 articles were included in this systematic review [2, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51].
Table 1 describes the characteristics of the PIM lists evaluated in this review. Most studies were conducted in Europe [2, 23, 25, 26, 28,29,30, 35, 36, 38, 39, 42, 43, 47, 49] and North America [17,18,19, 21, 22, 27, 32, 37, 41, 45, 46, 48, 51]. However, other countries from Asia [24, 31, 33, 34, 40, 50], Oceania [20], and South America [44] such as Taiwan [24], Pakistan [40], South Korea [33, 34], Thailand [50], Japan [31] Australia [20], and Chile [44] have also published lists of PIMs.
The majority of the PIM lists (23 PIM list, 63.9%) are aimed at the general population aged 65 years and older. The Norwegian General Practice (NORGEP) criteria [47] and its adaptation for nursing home residents were designed especially for individuals aged 70 years and older [42] and the French criteria [36] for those aged 75 years and older. Only three PIM lists (8.3%) were developed for nursing home residents [22, 32, 42], two (5.5%) for older hospitalized patients [28, 40] and one (2.7%) for use in community pharmacies [49].
Some PIM lists, such as Beers (1991, 1997, 2003, 2012 and 2015) [17, 18, 21, 22, 27], STOPP (Screening Tool of Older People’s Prescriptions) version 1 [29] (2008) and 2 (2015) [43], FORTA (Fit fOR The Aged) [35], Australian Prescribing Indicators Tool (2012) [20], Thailand criteria (2008) [50], and Lindblad criteria (2006) [37], used the current literature on efficacy and safety in older adults as an evidence base to develop their own list of PIMs. Other PIM lists, such as the McLeod criteria (1997) [41], Rancourt criteria (2004) [46], French criteria (2007) [36], NORGEP criteria (2009) [47], and PRISCUS (2010) [30], combined ECs previously published with a review of current literature. However, most PIM lists used previously published PIM lists to develop their lists of PIMs [2, 19, 23–26, 28, 31–34, 38–40, 42, 44–45, 48–49, 51]. Twenty-one (58.3%) of the 36 PIM lists were based on the Beers criteria and its updates, ten(27.8%) on the STOPP criteria and its update, and seven (19.4%) on the McLeod criteria. The tool developed by Tommelein et al. [49] was based on items derived from 14 different PIM lists (Table 1).
Of the 36 studies identified, 19 (52.8%) used the Delphi method and 14 (38.9%) used a modified Delphi method, to validate their ECs. Two studies used the RAND/UCLA [20, 49] (Research and Development/University of California, Los Angeles) process and the Italian criteria [38] used the Nominal Group Technique. The number of experts ranged from 4 to 62 and approximately 50.0% of the consensus panels included between 10 and 20 respondents. We observed a predominance of physicians and pharmacists whose practices concentrate on older adults and clinical pharmacology among the experts. Some studies included experts from different specialties such as psychiatrists [24, 30, 33, 38], cardiologists [24, 38], pulmonologists, gastroenterologists [24, 38], and urologists [24, 38] (Table 1).
Classification systems for PIMs varied between the studies. The majority of PIM lists provide an explicit listing of individual drugs. Eleven (30.6%) tools focused on PIMs to avoid in older adults independent of disease/condition; 22 (61.1%) included PIMs to avoid in older adults for specific diseases or conditions, and 20 (55.6%) mentioned relevant drug–drug interactions. Fourteen (38.8%) tools presented alternative therapies and 10 (27.8%) provided information about special considerations of use. Furthermore, 29 PIM lists (80.5%) also described doses or durations of medications, which should not be exceeded. Avoiding unnecessary duplication was mentioned in eight PIM lists (22.2%) (Table 2).
The 36 PIM lists identified a total of 907 different medications/ medication classes. Among them, only 4 classes and 44 medications were reported by more than 69% of PIM lists. The most prevalent class of medication identified as inappropriate was benzodiazepines, which were included in 33 (91.7%) of the 36 PIM lists. Other medication classes of PIMs identified included nonsteroidal anti-inflammatory drugs (NSAIDs) (28/36 PIM lists; 77.8%) followed by tricyclic antidepressants and antihistamines (27/36 PIM lists, 75.0%) (Table 3). Regarding the medications, only diazepam, chlordiazepoxide, indomethacin, and amitriptyline were considered inappropriate by 35 (97.2%) of the 36 PIM lists (Table 4).
Similar results were observed when we considered the 33 PIM lists (91.7%) that evaluated PIM independent of disease/condition. Benzodiazepines (29/33 PIM lists, 87.9%) and antihistamines (23/33 PIM lists, 69.7%) were the most common medication classes reported followed by tricyclic antidepressants (19/33 PIM lists, 57.6%) (Table 3). The commonest medications included were diazepam, chlordiazepoxide (31/33 PIM lists, 93.9%), amitriptyline, and chlorpheniramine (28/33 PIM lists, 84.8%) (Table 4).
Medications that can be avoided in individual diseases/conditions are specified in 22 PIM lists (61.1%). The most common medication classes implicated were NSAIDs (20/22 PIM lists, 90.9%), tricyclic antidepressants (19/ 22 PIM lists, 86.4%), followed by urologic spasmolytics, and long-acting benzodiazepines (18/22 PIM lists, 81.8%) (Table 3). Oxybutynin, diazepam, and chlordiazepoxide were the most common medications; they were reported as PIMs in specific diseases/conditions in 20 (90.9%) of 22 PIM lists (Table 4).
We identified 536 different drug–disease interactions involving 84 diseases/conditions. Among them, only 38 (7.0%) drug–disease interactions were cited in more than 25% of PIM lists. The most common conditions cited were constipation/chronic constipation (42/536 drug–disease interactions, 7.8%), dementia/cognitive impairment (41/536 drug–disease interactions, 7.6%), insomnia (36/536 drug–disease interactions, 6.7%), lower urinary tract symptoms/benign prostatic hyperplasia (28/536 drug–disease interactions, 5.2%), heart failure (19/536 drug–disease interactions, 3.5%), and history of falls/fractures (19/536 drug–disease interactions,3.5%). Table 5 summarizes the most common drug–disease interactions identified. The use of NSAIDs in patients with renal insufficiency (15/22 PIM lists, 68.1%) and heart failure (13/22 PIM lists, 59.1%)) and the use of metoclopramide in patients with Parkinson’s disease (13/22 PIM lists, 59.1%) were the most commonly reported. Other prevalent drug–disease interactions included anticholinergic drugs in those with dementia/cognitive impairment (12/22 PIM lists, 54.5%), benzodiazepines in those with a history of falls/fractures (11/22 PIM lists, 50.0%), and urologic spasmolytics in those with lower urinary tract symptoms/benign prostatic hyperplasia (10/22 PIM lists, 45.4%).
We also identified 159 potential drug–drug interactions described in 20 PIM lists. Among them, only 16 (10.1%) drug–drug interactions were cited in more than 20% of PIM lists. The most common medication classes implicated were the NSAIDs (19/20 PIM lists, 95.0%), tricyclic antidepressants (14/20 PIM lists, 70.0%), followed by angiotensin-converting-enzyme inhibitors (12/20 PIM lists, 55.6%), and selective serotonin reuptake inhibitors (10/20 PIM lists, 50.0%). Regarding single medications, warfarin was the most common medication reported; it was included in 18(90.0%) of 20 PIM lists that evaluated drug–drug interactions. Table 6 describes the most common drug–drug interactions described. The concomitant use of warfarin with NSAIDs (11/20 PIM lists, 55.5%) and aspirin (7/20 PIM lists, 35.0%) was the most common drug–drug interactions reported followed by the concomitant use of NSAIDs and ACE inhibitors (7/20 PIM lists, 35.0%) and the concomitant use of beta blockers and verapamil (7/20 PIM lists, 35.0%).
Discussion
This systematic review presents data from 36 PIM lists (published between 1991 and April 2017) that developed and validated EC for identification of PIMs. The aggregation and comparison of studies showed a wide variability of PIMs, and we identified different 907 medications/medication classes reported in all PIM lists. A previous systematic review identified 729 different medications/classes described in 14 different PIM lists published between 2006 and 2015 [57]. The higher number of medications/classes in our review is justified by the fact that we included more years and other baseline data in our search strategies, and we did not exclude PIM lists for institutionalized or hospitalized patients or criteria that reported only drug–disease interactions. Furthermore, aspects such as different settings and prescribing cultures, differences in medication availability/formulary between countries, and ethnopharmacology may have contributed to these results.
Conversely, we observed that less than half of PIM lists developed their own EC based on literature reviews. The development of evidence-based PIM lists is a dynamic and complex process, because older participants are commonly excluded from well-designed clinical trials [52, 53]. Thus, a majority of the studies used prior PIM lists to develop their own lists of PIMs [2, 19, 23–26, 28, 31–34, 38–40, 42, 44–45, 48–49, 51]. However, some of these authors have combined different PIM lists with drug references [2], pharmacoepidemiologic studies [25, 28, 33, 44], or prescribing indicators [28, 49] in order to include some medications and other instances of PIM use in older people (e.g., drug–drug interactions, drug class prescription duplication, special considerations of use, alternative therapies) which were not described in prior PIM lists.
We also verified that the majority of studies were developed for general practice. Few PIM lists focused on specific populations such as nursing home residents [22, 32, 42] and hospitalized patients [28, 40]. These were adaptations from existing PIM lists and included some new PIMs in their evaluation. For instance, some lists did not account for drugs frequently used during inpatient stays such as antibiotics. Thus, this result suggests that more work is needed to develop PIM lists for these populations and that some PIM lists originally designed for general practice could later be externally validated in these settings.
The Delphi technique was used to validate EC in the majority of the studies. This method is defined as an exercise in group communication that brings together and synthesizes the knowledge of a group of geographically distributed participants who have never meet [59]. Although there is no agreement on the definition of an expert, number of experts used, the number of rounds, and the consensus level in the literature, Delphi technique has some advantages over other consensus techniques such as the lack of discussion domination by any one panel member [60]. However, in this review, some studies [17, 18, 21, 27] modified the Delphi technique; these studies used a physical panel meeting at the end of consensus procedure in order to exchange views and resolve uncertainties.
We found that benzodiazepines and NSAIDs were the most common drugs reported as PIMs for older adults in all PIM lists. Previous systematic review also verified that these medication classes are among the most common reported in PIM lists [57]. However, these authors considered the number of indications of each medication class in each PIM lists while we evaluated the medication class included in each PIM lists. Of the 36 PIM lists evaluated, 33 described benzodiazepines as inappropriate. There is good observational data on the association between the use of benzodiazepine by older adults and serious ADEs, including impaired cognitive function [61, 62], delirium [63], respiratory insufficiency [64], falls [65], and fall-related injuries such as hip fractures [66]. Thus, they have the potential to create serious public health problems including hospitalization and death. Despite these risks, benzodiazepines are commonly used in the treatment of anxiety, depression, and insomnia in older patients around the world. Patients and providers hesitate to discontinue benzodiazepines because of the fear of withdrawal symptoms or relapse [67]. Studies show that there is a high prevalence of long-term use of this class in this age group, ranging 12 to 43% [68, 69].
There was very limited overlap between the PIM lists that we described in this study. Among all PIMs, only diazepam, chlordiazepoxide, indomethacin, and amitriptyline were considered inappropriate by 35 of the 36 PIM lists. Furthermore, only 44 medications and 4 medication classes were present in 69.0% or more of PIM lists. Prior systematic reviews also reported that only a few drugs are common to all the lists of PIMs published [57]. The heterogeneity in the lists of medications reflects the fact that medication management in older adults is extremely complex with a very limited evidence base to guide it. Additionally, health professionals from various fields were involved in the development of the PIM lists and they would, therefore, have different approaches and attitudes. As a consequence, the list of medications can vary widely.
We compiled all drug–disease interactions and drug–drug interactions included in the different PIM lists. It is interesting to note that NSAIDs were the most common medication class in both types of drug interactions. Despite the consistent recommendations to avoid the use of this medication class in different situations, it is estimated that 40% of people aged 65 years and older fill one or more prescriptions for a NSAIDs each year [70] with additional users accessing NSAIDs over the counter [71]. This, like the high utilization of benzodiazepines, may highlight the limited impact of the consensus on PIMs or that, while potentially inappropriate, the benefit may frequently be determined to outweigh the risk for the individual.
We identified the drug–drug interactions described in 20 PIM lists. Although a considerable proportion of adverse drug reactions is caused by interactions between drugs [72, 73], drug–drug interactions are still underreported in the criteria for assessing inappropriate prescriptions in older adults. Of the 159 drug–drug interactions identified, only 16 are described in more than 20% of the PIM lists. The concomitant use of NSAIDs and aspirin with warfarin was the most frequent drug–drug interaction described. Many studies have provided an increased risk of hospitalization in elderly adults using this combination of drugs [72]. Additionally, the warfarin was the most common single medication reported among the drug–drug interactions lists. Despite this medication is highly effective in the prevention of stroke in atrial fibrillation, it is known for its interaction with many drugs [72–73], which is the leading cause of adverse drug event-related hospitalizations in older adults and can lead to fatal outcomes in this population [74].
Strengths
This is the first study that systematically compiled all drug–disease interactions and drug–drug interactions included in validated PIM lists since 1991. This systematic review used a comprehensive search strategy applied by the reviewers without language limitations. Furthermore, the study followed the PRISMA methodology, including study selection performed by two independent reviewers with arbitration by a third party if necessary. This reduced the risk of studies being omitted and also reduced the risk of selection bias.
Limitations
Our review had some important limitations. EC are limited in that they do not address individual differences among patients or the complexity or appropriateness of entire medication regimens. Furthermore, they need to be regularly updated in line with the evidence, and country-specific adaptations are necessary where countries differ in their guidelines, standards, and approved medications. It is important to recognize that a detailed description of the consensus method was not included in some studies [26, 42, 43]. To our knowledge, there is no formal method for quality assessment or risk of bias for consensus studies, so a rigorous assessment of the quality/bias of each study could not be performed as required by the PRISMA criteria [58].
Conclusion
Appropriate mediation management among older adults can help prevent serious adverse drug events [3, 10] which are associated with the increase of hospitalization and mortality in this population. For this reason, approaches aimed at detecting inappropriate prescriptions have intensified in the last decades with the development and validation of a number of strategies, particularly PIM lists. These PIM lists are important educational tools and should be included in the comprehensive assessment of every older patient who requires medication. We identified 36 different PIM lists. Different medication/medication classes, drug–disease interactions, and drug–drug interactions were included in different lists, with limited overlap between the PIM lists presented. These results demonstrate that the use of medications in older people is complex field and that more evidence is required to be able to generate consistent expert recommendations and to implement them.
Our review highlights the most common PIMs, drug–disease interactions, and drug–drug interactions validated by expert consensus for over 26 years. These results can help health professionals to elaborate strategies to minimize use of PIMS in many different settings. Although benzodiazepines and NSAIDs were the most common medications classified as being inappropriate, they are still commonly used in older adults. Avoiding medication in which the risks outweigh the benefits in the elderly patient continues to be a challenge for health professionals. Some PIM lists are complex and did not provide special considerations of use and alternative medications to avoid those considered potentially inappropriate. In addition, few PIM lists provide information that supports safely tapering or withdrawing PIM. These facts may compromise the use of PIM lists in clinical practice. Future PIM lists should integrate information about alternative therapies and special considerations of use in order to help clinicians to make decisions about drug prescription.
References
Dimitrow MS, Airaksinen MS, Kivela SL, Lyles A, Leikola SN (2011) Comparison of prescribing criteria to evaluate the appropriateness of drug treatment in individuals aged 65 and older: a systematic review. J Am Geriatr Soc 59(8):1521–1530. https://doi.org/10.1111/j.1532-5415.2011.03497.x
Renom-Guiteras A, Meyer G, Thurmann PA (2015) The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 71(7):861–875. https://doi.org/10.1007/s00228-015-1860-9
Hedna K, Hakkarainen KM, Gyllensten H, Jonsson AK, Petzold M, Hagg S (2015) Potentially inappropriate prescribing and adverse drug reactions in the elderly: a population-based study. Eur J Clin Pharmacol 71(12):1525–1533. https://doi.org/10.1007/s00228-015-1950-8
Morin L, Laroche ML, Texier G, Johnell K (2016) Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc 17(9):862.e861–862.e869. https://doi.org/10.1016/j.jamda.2016.06.011
Nyborg G, Straand J, Brekke M (2012) Inappropriate prescribing for the elderly—a modern epidemic? Eur J Clin Pharmacol 68(7):1085–1094. https://doi.org/10.1007/s00228-012-1223-8
Opondo D, Eslami S, Visscher S, de Rooij SE, Verheij R, Korevaar JC, Abu-Hanna A (2012) Inappropriateness of medication prescriptions to elderly patients in the primary care setting: a systematic review. PLoS One 7(8):e43617. https://doi.org/10.1371/journal.pone.0043617
Corsonello A, Pedone C, Incalzi RA (2010) Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem 17(6):571–584. https://doi.org/10.2174/092986710790416326
Mangoni AA, Jackson SH (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 57(1):6–14. https://doi.org/10.1046/j.1365-2125.2003.02007.x
Hanlon JT, Shimp LA, Semla TP (2000) Recent advances in geriatrics: drug-related problems in the elderly. Ann Pharmacother 34(3):360–365. https://doi.org/10.1345/aph.19140
Lund BC, Carnahan RM, Egge JA, Chrischilles EA, Kaboli PJ (2010) Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother 44(6):957–963. https://doi.org/10.1345/aph.1M657A
Cabre M, Elias L, Garcia M, Palomera E, Serra-Prat M (2017) Avoidable hospitalizations due to adverse drug reactions in an acute geriatric unit. Analysis of 3,292 patients. Med Clin. https://doi.org/10.1016/j.medcli.2017.06.075
Price SD, Holman CD, Sanfilippo FM, Emery JD (2014) Association between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patients. Ann Pharmacother 48(1):6–16. https://doi.org/10.1177/1060028013504904
Reich O, Rosemann T, Rapold R, Blozik E, Senn O (2014) Potentially inappropriate medication use in older patients in Swiss managed care plans: prevalence, determinants and association with hospitalization. PLoS One 9(8):e105425. https://doi.org/10.1371/journal.pone.0105425
Klarin I, Wimo A, Fastbom J (2005) The association of inappropriate drug use with hospitalisation and mortality: a population-based study of the very old. Drugs Aging 22(1):69–82
Lau DT, Kasper JD, Potter DE, Lyles A, Bennett RG (2005) Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med 165(1):68–74. https://doi.org/10.1001/archinte.165.1.68
Muhlack DC, Hoppe LK, Weberpals J, Brenner H, Schottker B (2017) The association of potentially inappropriate medication at older age with cardiovascular events and overall mortality: a systematic review and meta-analysis of cohort studies. J Am Med Dir Assoc 18(3):211–220. https://doi.org/10.1016/j.jamda.2016.11.025
American Geriatrics Society Beers Criteria Update Expert P (2012) American Geriatrics Society updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 60(4):616–631. https://doi.org/10.1111/j.1532-5415.2012.03923.x
American Geriatrics Society Beers Criteria Update Expert P (2015) American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 63(11):2227–2246. https://doi.org/10.1111/jgs.13702
Bachyrycz A, Dodd MA, Priloutskaya G (2012) Development and dissemination of a statewide system to minimize use of potentially inappropriate medications (PIMs). Med Care 50(11):993–996. https://doi.org/10.1097/MLR.0b013e31826ecfdc
Basger BJ, Chen TF, Moles RJ (2012) Validation of prescribing appropriateness criteria for older Australians using the RAND/UCLA appropriateness method. BMJ Open 2(5):e001431. https://doi.org/10.1136/bmjopen-2012-001431
Beers MH (1997) Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med 157(14):1531–1536
Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC (1991) Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch Intern Med 151(9):1825–1832
Castillo-Paramo A, Pardo-Lopo R, Gomez-Serranillos IR, Verdejo A, Figueiras A, Claveria A (2013) Assessment of the appropriateness of STOPP/START criteria in primary health care in Spain by the RAND method. SEMERGEN 39(8):413–420. https://doi.org/10.1016/j.semerg.2013.01.017
Chang CB, Yang SY, Lai HY, Wu RS, Liu HC, Hsu HY, Hwang SJ, Chan DC (2012) Using published criteria to develop a list of potentially inappropriate medications for elderly patients in Taiwan. Pharmacoepidemiol Drug Saf 21(12):1269–1279. https://doi.org/10.1002/pds.3274
Clyne B, Bradley MC, Hughes CM, Clear D, McDonnell R, Williams D, Fahey T, Smith SM, O-Ss t (2013) Addressing potentially inappropriate prescribing in older patients: development and pilot study of an intervention in primary care (the OPTI-SCRIPT study). BMC Health Serv Res 13:307. https://doi.org/10.1186/1472-6963-13-307
Fialova DT, Topinkova E, Ballokova A, Matejovska-Kubesova H (2013) 2012 CZ expert consensus for potentially inappropriate medication use in old age: appropriate choice of drugs and drug dosing in geriatric patients (section I), drug-disease interactions in the old age (Section II). Klin Farmakol Farmacie 27(1):18–28
Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH (2003) Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 163(22):2716–2724. https://doi.org/10.1001/archinte.163.22.2716
Galan Retamal C, Garrido Fernandez R, Fernandez Espinola S, Ruiz Serrato A, Garcia Ordonez MA, Padilla Marin V (2014) Prevalence of potentially inappropriate medication in hospitalized elderly patients by using explicit criteria. Farm Hosp 38(4):305–316. https://doi.org/10.7399/fh.2014.38.4.1148
Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D (2008) STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 46(2):72–83
Holt S, Schmiedl S, Thurmann PA (2010) Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int 107(31–32):543–551. https://doi.org/10.3238/arztebl.2010.0543
Imai HB, MH FDM (2008) Development of Japanese Beers criteria for inappropriate medication use in elderly patients. Jpn Med Assoc J 137:1348–1358
Khodyakov D, Ochoa A, Olivieri-Mui BL, Bouwmeester C, Zarowitz BJ, Patel M, Ching D, Briesacher B (2017) Screening Tool of Older Person's Prescriptions/Screening Tools to Alert Doctors to Right Treatment medication criteria modified for U.S. nursing home setting. J Am Geriatr Soc 65(3):586–591. https://doi.org/10.1111/jgs.14689
Kim DS, Heo SI, Lee SH (2010) Development of a list of potentially inappropriate drugs for the korean elderly using the Delphi method. Healthc Inf Res 16(4):231–252. https://doi.org/10.4258/hir.2010.16.4.231
Kim S-O, Jang S, Kim C-M, Kim Y-R, Sohn HS (2015) Consensus validated list of potentially inappropriate medication for the elderly and their prevalence in South Korea. Int J Gerontol 9(3):136–141. https://doi.org/10.1016/j.ijge.2015.05.013
Kuhn-Thiel AM, Weiss C, Wehling M, Faep m (2014) Consensus validation of the FORTA (Fit fOR The Aged) List: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging 31(2):131–140. https://doi.org/10.1007/s40266-013-0146-0
Laroche ML, Charmes JP, Merle L (2007) Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol 63(8):725–731. https://doi.org/10.1007/s00228-007-0324-2
Lindblad CI, Hanlon JT, Gross CR, Sloane RJ, Pieper CF, Hajjar ER, Ruby CM, Schmader KE (2006) Clinically important drug-disease interactions and their prevalence in older adults. Clin Ther 28(8):1133–1143. https://doi.org/10.1016/j.clinthera.2006.08.006
Maio V, Del Canale S, Abouzaid S, Investigators GAP (2010) Using explicit criteria to evaluate the quality of prescribing in elderly Italian outpatients: a cohort study. J Clin Pharm Ther 35(2):219–229. https://doi.org/10.1111/j.1365-2710.2009.01094.x
Mann E, Bohmdorfer B, Fruhwald T, Roller-Wirnsberger RE, Dovjak P, Duckelmann-Hofer C, Fischer P, Rabady S, Iglseder B (2012) Potentially inappropriate medication in geriatric patients: the Austrian consensus panel list. Wien Klin Wochenschr 124(5–6):160–169. https://doi.org/10.1007/s00508-011-0061-5
Mazhar F, Akram S, Malhi SM, Haider N (2017) A prevalence study of potentially inappropriate medications use in hospitalized Pakistani elderly. Aging Clin Exp Res 30:53–60. https://doi.org/10.1007/s40520-017-0742-7
McLeod PJ, Huang AR, Tamblyn RM, Gayton DC (1997) Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ 156(3):385–391
Nyborg G, Straand J, Klovning A, Brekke M (2015) The Norwegian general practice—nursing home criteria (NORGEP-NH) for potentially inappropriate medication use: a web-based Delphi study. Scand J Prim Health Care 33(2):134–141. https://doi.org/10.3109/02813432.2015.1041833
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44(2):213–218. https://doi.org/10.1093/ageing/afu145
Passi A, Margozzini P, Valenzuela E, Hoyl T, Marin PP, Carrasco M, Olea R, Gac H (2016) Inappropriate medication use among Chilean older people. Rev Med Chil 144(4):417–425. https://doi.org/10.4067/S0034-98872016000400001
Pugh MJ, Hanlon JT, Zeber JE, Bierman A, Cornell J, Berlowitz DR (2006) Assessing potentially inappropriate prescribing in the elderly Veterans Affairs population using the HEDIS 2006 quality measure. J Manag Care Pharm 12(7):537–545. https://doi.org/10.18553/jmcp.2006.12.7.537
Rancourt C, Moisan J, Baillargeon L, Verreault R, Laurin D, Gregoire JP (2004) Potentially inappropriate prescriptions for older patients in long-term care. BMC Geriatr 4:9. https://doi.org/10.1186/1471-2318-4-9
Rognstad S, Brekke M, Fetveit A, Spigset O, Wyller TB, Straand J (2009) The Norwegian General Practice (NORGEP) criteria for assessing potentially inappropriate prescriptions to elderly patients. A modified Delphi study. Scand J Prim Health Care 27(3):153–159. https://doi.org/10.1080/02813430902992215
Stuck AE, Beers MH, Steiner A, Aronow HU, Rubenstein LZ, Beck JC (1994) Inappropriate medication use in community-residing older persons. Arch Intern Med 154(19):2195–2200
Tommelein E, Mehuys E, Petrovic M, Somers A, Van Damme C, Pattyn E, Mattelin K, Boussery K (2016) Potentially inappropriate prescribing in nursing home residents detected with the community pharmacist specific GheOP(3)S-tool. Int J Clin Pharm 38(5):1063–1068. https://doi.org/10.1007/s11096-016-0366-6
Winit-Watjana W, Sakulrat P, Kespichayawattana J (2008) Criteria for high-risk medication use in Thai older patients. Arch Gerontol Geriatr 47(1):35–51. https://doi.org/10.1016/j.archger.2007.06.006
Zhan C, Sangl J, Bierman AS, Miller MR, Friedman B, Wickizer SW, Meyer GS (2001) Potentially inappropriate medication use in the community-dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey. JAMA 286(22):2823–2829
Cherubini A, Oristrell J, Pla X, Ruggiero C, Ferretti R, Diestre G, Clarfield AM, Crome P, Hertogh C, Lesauskaite V, Prada GI, Szczerbinska K, Topinkova E, Sinclair-Cohen J, Edbrooke D, Mills GH (2011) The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med 171(6):550–556. https://doi.org/10.1001/archinternmed.2011.31
Crome P, Lally F, Cherubini A, Oristrell J, Beswick AD, Clarfield AM, Hertogh C, Lesauskaite V, Prada GI, Szczerbinska K, Topinkova E, Sinclair-Cohen J, Edbrooke D, Mills G (2011) Exclusion of older people from clinical trials: professional views from nine European countries participating in the PREDICT study. Drugs Aging 28(8):667–677. https://doi.org/10.2165/11591990-000000000-00000
Marriott J, Stehlik P (2012) A critical analysis of the methods used to develop explicit clinical criteria for use in older people. Age Ageing 41(4):441–450. https://doi.org/10.1093/ageing/afs064
Chang CB, Chan DC (2010) Comparison of published explicit criteria for potentially inappropriate medications in older adults. Drugs Aging 27(12):947–957. https://doi.org/10.2165/11584850-000000000-00000
Kaufmann CP, Tremp R, Hersberger KE, Lampert ML (2014) Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol 70(1):1–11. https://doi.org/10.1007/s00228-013-1575-8
Lucchetti G, Lucchetti AL (2017) Inappropriate prescribing in older persons: a systematic review of medications available in different criteria. Arch Gerontol Geriatr 68:55–61. https://doi.org/10.1016/j.archger.2016.09.003
Duran CE, Azermai M, Vander Stichele RH (2013) Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol 69(7):1485–1496. https://doi.org/10.1007/s00228-013-1499-3
Dalkey NC (1969) The Delphi method: an experimental study of group opinion. Santa Monica, CA: RAND Corporation. Available at: http://www.rand.org/pubs/research_memoranda/RM5888
Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C (2011) Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 6(6):e20476. https://doi.org/10.1371/journal.pone.0020476
Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen PA, Poly TN, Masud JH, Li YJ, Shabbir SA (2016) Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology 47(3–4):181–191. https://doi.org/10.1159/000454881
Zhong G, Wang Y, Zhang Y, Zhao Y (2015) Association between benzodiazepine use and dementia: a meta-analysis. PLoS One 10(5):e0127836. https://doi.org/10.1371/journal.pone.0127836
Clegg A, Young JB (2011) Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing 40(1):23–29. https://doi.org/10.1093/ageing/afq140
Vozoris NT, Fischer HD, Wang X, Anderson GM, Bell CM, Gershon AS, Stephenson AL, Gill SS, Rochon PA (2013) Benzodiazepine use among older adults with chronic obstructive pulmonary disease: a population-based cohort study. Drugs Aging 30(3):183–192. https://doi.org/10.1007/s40266-013-0056-1
Leipzig RM, Cumming RG, Tinetti ME (1999) Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc 47(1):40–50
Finkle WD, Der JS, Greenland S, Adams JL, Ridgeway G, Blaschke T, Wang Z, Dell RM, VanRiper KB (2011) Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc 59(10):1883–1890. https://doi.org/10.1111/j.1532-5415.2011.03591.x
Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S (2014) Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 174(6):890–898. https://doi.org/10.1001/jamainternmed.2014.949
Cheng JS, Huang WF, Lin KM, Shih YT (2008) Characteristics associated with benzodiazepine usage in elderly outpatients in Taiwan. Int J Geriatr Psychiatry 23(6):618–624. https://doi.org/10.1002/gps.1950
Johnson CF, Frei C, Downes N, McTaggart SA, Akram G (2016) Benzodiazepine and z-hypnotic prescribing for older people in primary care: a cross-sectional population-based study. Br J Gen Pract 66(647):e410–e415. https://doi.org/10.3399/bjgp16X685213
Ray WA, Stein CM, Byrd V, Shorr R, Pichert JW, Gideon P, Arnold K, Brandt KD, Pincus T, Griffin MR (2001) Educational program for physicians to reduce use of non-steroidal anti-inflammatory drugs among community-dwelling elderly persons: a randomized controlled trial. Med Care 39(5):425–435
Marcum ZA, Hanlon JT (2010) Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann Longterm Care 18(9):24–27
Hines LE, Murphy JE (2011) Potentially harmful drug-drug interactions in the elderly: a review. Am J Geriatr Pharmacother 9(6):364–377. https://doi.org/10.1016/j.amjopharm.2011.10.004
Marengoni A, Pasina L, Concoreggi C, Martini G, Brognoli F, Nobili A, Onder G, Bettoni D (2014) Understanding adverse drug reactions in older adults through drug-drug interactions. Eur J Intern Med 25(9):843–846. https://doi.org/10.1016/j.ejim.2014.10.001
Budnitz DS, Lovegrove MC, Shehab N, Richards CL (2011) Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 365(21):2002–2012. https://doi.org/10.1056/NEJMsa1103053
Acknowledgements
We thank to the Coordination for the Improvement of Higher Education Personnel, National Council for Scientific and Technological Development for the support that they are providing for development of this study.
Funding
FRM was supported by the Coordination for the Improvement of Higher Education Personnel—CAPES through a doctorate at University of Vale do Rio dos Sinos, Brazil. FRM was also supported by CAPES through a sandwich doctorate fellowship at University of Sydney, Australia (number grant: 88881.134589/2016-01). This systematic review was funded by the National Council for Scientific and Technological Development-CNPQ (number grant: 426720/2016-4). The funders were not involved in the design or conduct of the study, collection, analysis, or interpretation of the data or preparation or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
FRM and VPM participated in all stages of this project, from the design and interpretation of data to its final writing. FRM and JSF conducted the development of search strategies, selection procedure, data extraction, data synthesis, and analysis. EVP contributed to the database organization and data extraction. SNH contributed to the critical review and writing of this manuscript. All authors participated in the discussions, result interpretation, and approved the final version of manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Appendix 1
(DOC 63 kb)
Appendix 2
(DOCX 72 kb)
Rights and permissions
About this article
Cite this article
Motter, F.R., Fritzen, J.S., Hilmer, S.N. et al. Potentially inappropriate medication in the elderly: a systematic review of validated explicit criteria. Eur J Clin Pharmacol 74, 679–700 (2018). https://doi.org/10.1007/s00228-018-2446-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2446-0