Abstract
Purpose
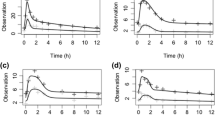
A pharmacokinetic (PK) model is available for describing the time course of the concentrations of methotrexate (MTX or MTXGlu1) and its active polyglutamated metabolites (MTXGlu2–5) in red blood cells (RBCs). In this study, we aimed to simplify the MTX PK model and to optimise the blood sampling schedules for use in future studies.
Methods
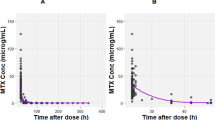
A proper lumping technique was used to simplify the original MTX RBC PK model. The sum of predicted RBC MTXGlu3–5 concentrations in both the simplified and original models was compared. The sampling schedules for MTXGlu3–5 or all MTX polyglutamates in RBCs were optimised using the Population OPTimal design (POPT) software.
Results
The MTX RBC PK model was simplified into a three-state model. The maximum of the absolute value of relative difference in the sum of predicted RBC MTXGlu3–5 concentrations over time was 6.3 %. A five blood sample design was identified for estimating parameters of the simplified model.
Conclusions
This study illustrates the application of model simplification processes to an existing model for MTX RBC PK. The same techniques illustrated in our study may be adopted by other studies with similar interest.




Similar content being viewed by others
References
Lambert CM, Sandhu S, Lochhead A, Hurst NP, McRorie E, Dhillon V (2004) Dose escalation of parenteral methotrexate in active rheumatoid arthritis that has been unresponsive to conventional doses of methotrexate: a randomized, controlled trial. Arthritis Rheum 50:364–371
Halilova KI, Brown EE, Morgan SL, Bridges SL Jr, Hwang MH, Arnett DK, Danila MI (2012) Markers of treatment response to methotrexate in rheumatoid arthritis: where do we stand? Int J Rheumatol 2012:978396–978402
Korell J, Stamp LK, Barclay ML, Dalrymple JM, Drake J, Zhang M, Duffull SB (2013) A population pharmacokinetic model for low-dose methotrexate and its polyglutamated metabolites in red blood cells. Clin Pharmacokinet 52:475–485
Okino MS, Mavrovouniotis ML (1998) Simplification of mathematical models of chemical reaction systems. Chem Rev 98:391–408
Matherly LH, Goldman DI (2003) Membrane transport of folates. Vitam Horm 66:403–456
Brooks AJ, Begg EJ, Zhang M, Frampton CM, Barclay ML (2007) Red blood cell methotrexate polyglutamate concentrations in inflammatory bowel disease. Ther Drug Monit 29:619–625
Pan S, Stamp L, Duffull S, Barclay M, Dalrymple J, Drake J, Zhang M, Korell J (2014) Assessment of the relationship between methotrexate polyglutamates in red blood cells and clinical response in patients commencing methotrexate for rheumatoid arthritis. Clin Pharmacokinet 53:1161–1170
Foo LK, McGree J, Duffull S (2012) A general method to determine sampling windows for nonlinear mixed effects models with an application to population pharmacokinetic studies. Pharm Stat 11:325–333
Wei J, Kuo JCW (1969) Lumping analysis in monomolecular reaction systems. Analysis of the exactly lumpable system. Ind Eng Chem Fundam 8:114–123
Dokoumetzidis A, Aarons L (2009) Proper lumping in systems biology models. IET Syst Biol 3:40–51
Gulati A, Isbister GK, Duffull SB (2014) Scale reduction of a systems coagulation model with an application to modeling pharmacokinetic-pharmacodynamic data. CPT Pharmacometrics Syst Pharmacol 3, e90
Dalrymple JM, Stamp LK, O'Donnell JL, Chapman PT, Zhang M, Barclay ML (2008) Pharmacokinetics of oral methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 58:3299–3308
Stamp LK, Barclay ML, O'Donnell JL, Zhang M, Drake J, Frampton C, Chapman PT (2011) Effects of changing from oral to subcutaneous methotrexate on red blood cell methotrexate polyglutamate concentrations and disease activity in patients with rheumatoid arthritis. J Rheumatol 38:2540–2547
Shivva V, Korell J, Tucker IG, Duffull SB (2013) An approach for identifiability of population pharmacokinetic-pharmacodynamic models. CPT Pharmacometrics Syst Pharmacol 2, e49
Duffull SB, Denman N, Kimko H, Eccleston J (2012) Population OPTimal design. In POPT—installation and user guide. Available from: http://www.winpopt.com/
Al-Sallami H, Cheah S, Han S, Liew J, Lim J, Ng M, Solanki H, Soo R, Tan V, Duffull S (2014) Between-subject variability: should high be the new normal? Eur J Clin Pharmacol 70:1403–1404
Nyberg J, Bazzoli C, Ogungbenro K, Aliev A, Leonov S, Duffull S, Hooker AC, Mentre F (2015) Methods and software tools for design evaluation in population pharmacokinetics-pharmacodynamics studies. Br J Clin Pharmacol 79:6–17
Bellu G, Saccomani MP, Audoly S, D'Angio L (2007) DAISY: a new software tool to test global identifiability of biological and physiological systems. Comput Methods Prog Biomed 88:52–61
D'Argenio D (1981) Optimal sampling times for pharmacokinetic experiments. J Pharmacokinet Biopharm 9:739–756
Foo LK, Duffull SB (2011) Optimal design of pharmacokinetic–pharmacodynamic studies. In: Bonate PL, Howard DR (eds) Pharmacokinetics in drug development. Springer US, pp 175–193
Steven Ernest C 2nd, Nyberg J, Karlsson MO, Hooker AC (2014) Optimal clinical trial design based on a dichotomous Markov-chain mixed-effect sleep model. J Pharmacokinet Pharmacodyn 41:639–654
Acknowledgments
The clinical studies were supported by the Health Research Council of New Zealand and New Zealand Pharmacy Education and Research Foundation.
Shan Pan was supported by a University of Otago PhD scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
S.P., J.K., L.K.S., and S.B.D. wrote the manuscript. S.P., J.K., L.K.S., and S.B.D. designed the research. S.P. and S.B.D. performed the research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 250 kb)
Rights and permissions
About this article
Cite this article
Pan, S., Korell, J., Stamp, L.K. et al. Simplification of a pharmacokinetic model for red blood cell methotrexate disposition. Eur J Clin Pharmacol 71, 1509–1516 (2015). https://doi.org/10.1007/s00228-015-1951-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1951-7