Abstract
Context
Cisplatin, a coordination platinum complex, is used as a potential anti-neoplastic agent, having well recognized DNA-damaging property that triggers cell-cycle arrest and cell death in cancer therapy. Beneficial chemotherapeutic actions of cisplatin can be detrimental for kidneys.
Background
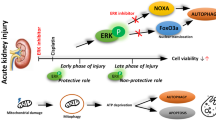
Unbound cisplatin gets accumulated in renal tubular cells, leading to cell injury and death. This liable action of cisplatin on kidneys is mediated by altered intracellular signalling pathways such as mitogen-activated protein kinase (MAPK), extracellular regulated kinase (ERK), or C- Jun N terminal kinase/stress-activated protein kinase (JNK/SAPK). Further, these signalling alterations are responsible for release and activation of tumour necrosis factor (TNF-α), mitochondrial dysfunction, and apoptosis, which ultimately cause the renal pathogenic process. Cisplatin itself enhances the generation of reactive oxygen species (ROS) and activation of nuclear factor-κB (NF-κB), inflammation, and mitochondrial dysfunction, which further leads to renal apoptosis. Cisplatin-induced nephropathy is also mediated through the p53 and protein kinase-Cδ (PKCδ) signalling pathways.
Objective
This review explores these signalling alterations and their possible role in the pathogenesis of cisplatin-induced renal injury.

Similar content being viewed by others
Abbreviations
- MAPKs:
-
Mitogen activated protein kinases
- ERK:
-
Extracellular regulated kinase
- JNK:
-
Jun N terminal kinase
- SAPKs:
-
Stress-activated protein kinases
- PKCδ:
-
Protein kinase-Cδ
- ROS:
-
Reactive oxygen species
- ATF3:
-
Activating transcription factor 3
- GPCRs:
-
G protein coupled receptors
- RTKs:
-
Receptor tyrosine kinases
- iNOS:
-
Inducible nitric oxide synthase
- TNF-α:
-
Tumour necrosis factor
- NF-κB:
-
Nuclear factor- κB
- PIDD:
-
p53-inducible death domain
- PUMA:
-
p53-upregulated modulator of apoptosis
- H2O2 :
-
Hydrogen peroxide
References
Wagner JM, Karnitz LM (2009) Cisplatin-induced DNA damage activates replication checkpoint signalling components that differentially affect tumor cell survival. Mol Pharmacol 76:208–14
Anand AJ, Bashey B (1993) Newer insights into cisplatin nephrotoxicity. Ann Pharmacother 27:1519–25
Fillastre JP, Raguenez-Viotte G (1989) Cisplatin nephrotoxicity. Toxicol Lett 46:163–75
Guerrero-Beltrán CE, Mukhopadhyay P, Horváth B, Rajesh M, Tapia E, García-Torres I, Pedraza-Chaverri J, Pacher P (2012) Sulforaphane, a natural constituent of broccoli, prevents cell death and inflammation in nephropathy. J Nutr Biochem 23:494–500
Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334:115–24
Pabla N, Murphy RF, Liu K, Dong Z (2009) The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol 296:505–11
Jo SK, Cho WY, Sung SA, Kim HK, Won NH (2005) MEK inhibitor, U0126 attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int 67:458–66
So H, Kim H, Lee JH, Park C, Kim Y, Kim E, Kim JK, Yun KJ, Lee KM, Lee HY, Moon SK, Lim DJ, Park R (2007) Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol 8:338–55
Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS, Jung JS, Kim JM (2005) Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol 25:374–82
Wang X, Martindale JL, Holbrook NJ (2000) Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem 275:39435–43
Arany I, Megyesi JK, Kaneto H, Price PM, Safirstein RL (2004) Cisplatin induced cell death is EGFR/src/ERK signalling dependent in mouse proximal tubule cells. Am J Physiol Renal Physiol 287:543–49
Deschesnes RG, Huot J, Valerie K, Landry J (2001) Involvement of p38 in apoptosis associated membrane blebbing and nuclear condensation. Mol Biol cell 12:1569–82
Hernández Losa J, Parada Cobo C, Guinea Viniegra J, Sánchez-Arevalo Lobo VJ, Ramón y Cajal S, Sánchez-Prieto R (2003) Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene 22:3998–4006
Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, Siddik ZH, Mills GB, Claret FX (2003) Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem 278:19245–56
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7:683–94
Gartel AL, Tyner AL (2002) The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1:639–49
Dimanche-Boitrel MT, Meurette O, Rebillard A, Lacour S (2005) Role of early plasma membrane events in chemotherapy-induced cell death. Drug Resist Updat 8:5–14
Kröning R, Lichtenstein AK, Nagami GT (2000) Sulfur-containing amino acids decrease cisplatin cytotoxicity and uptake in renal tubule epithelial cell lines. Cancer Chemother Pharmacol 45:43–49
Holzer AK, Samimi G, Katano K, Naerdemann W, Lin X, Safaei R, Howell SB (2004) The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol 66:817–23
Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z (2007) Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293:282–91
Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z (2011) Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest 121:2709–22
Pabla N, Dong Z (2012) Curtailing side effects in chemotherapy: a tale of PKCδ in cisplatin treatment. Oncotarget 3:107–11
Mukhopadhyay P, Horváth B, Zsengellér Z, Zielonka J, Tanchian G, Holovac E, Kechrid M, Patel V, Stillman IE, Parikh SM, Joseph J, Kalyanaraman B, Pacher P (2012) Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med 52:497–506
Tanabe K, Tamura Y, Lanaspa MA, Miyazaki M, Suzuki N, Sato W, Maeshima Y, Schreiner GF, Villarreal FJ, Johnson RJ, Nakagawa T (2012) Epicatechin limits renal injury by mitochondrial protection in cisplatin nephropathy. Am J Physiol Renal Physiol 303:264–74
Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Pérez JM (2007) Biochemical mechanisms of cisplatin cytotoxicity. Anti-Cancer Agents Med Chem 7:3–18
Gallo KA, Johnson GL (2002) Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3:663–72
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–44
Cano E, Mahadevan LC (1995) Parallel signal processing among mammalian MAPKs. Trends Biochem Sci 20:117–22
Su B, Karin M (1996) Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol 8:402–11
Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C (2012) Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signalling pathways. FASEB J 26:4805–20
Seger R, Krebs EG (1995) The MAPK signalling cascade. FASEB J 9:726–35
Wang J, Huang B, Xia X, Sun Z (2006) Funneled landscape leads to robustness of cellular networks: MAPK signal transduction. Biophys J 91:54–56
Ferrer I, Blanco R, Carmona M, Puig B (2001) Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J Neural Transm 108:1397–415
Wei L, Zhu Z, Wang J, Liu J (2009) JNK and p38 mitogen-activated protein kinase pathways contribute to porcine circovirus type 2 infection. J Virol 83:6039–47
Cassidy H, Radford R, Slyne J, O’Connell S, Slattery C, Ryan MP, McMorrow T (2012) The role of MAPK in drug-induced kidney injury. J Signal Transduct 2012:463617
Rubinfeld H, Seger R (2005) The ERK cascade: a prototype of MAPK signalling. Mol Biotechnol 31:151–74
Raman M, Chen W, Cobb MH (2007) Differential regulation and properties of MAPKs. Oncogene 26:3100–12
Keshet Y, Seger R (2010) The MAP kinase signalling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol 661:3–38
Arany I, Safirstein RL (2003) Cisplatin nephrotoxicity. Semin Nephrol 23:460–64
St Germain C, Niknejad N, Ma L, Garbuio K, Hai T, Dimitroulakos J (2010) Cisplatin induces cytotoxicity through the mitogen-activated protein kinase pathways and activating transcription factor 3. Neoplasia 12:527–38
Lu D, Chen J, Hai T (2007) The regulation of ATF3 gene expression by mitogen activated protein kinases. Biochem J 401:559–67
Inoue K, Zama T, Kamimoto T, Aoki R, Ikeda Y, Kimura H, Hagiwara M (2004) TNFalpha-induced ATF3 expression is bidirectionally regulated by the JNK and ERK pathways in vascular endothelial cells. Genes Cells 9:59–70
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73:994–1007
Blaukat A, Barac A, Cross MJ, Offermanns S, Dikic I (2000) G protein-coupled receptor-mediated mitogen-activated protein kinase activation through cooperation of Galpha(q) and Galpha(i) signals. Mol Cell Biol 20:6837–48
Wetzker R, Böhmer FD (2003) Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol 4:651–57
Bogoyevitch MA, Court NW (2004) Counting on mitogen-activated protein kinases–ERKs 3, 4, 5, 6, 7 and 8. Cell Signal 16:1345–54
Yoon S, Seger R (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24:21–44
Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W (2005) Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem J 392:249–61
Wortzel I, Seger R (2011) The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer 2:195–209
Choi BK, Choi CH, Oh HL, Kim YK (2004) Role of ERK activation in cisplatin induced apoptosis in A172 human glioma cells. Neurotoxicology 25:915–24
Tashker JS, Olson M, Kornbluth S (2002) Post-cytochrome C protection from apoptosis conferred by a MAPK pathway in Xenopus egg extracts. Mol Biol Cell 13:393–401
Park MC, Kang T, Jin D, Han JM, Kim SB, Park YJ, Cho K, Park YW, Guo M, He W, Yang XL, Schimmel P, Kim S (2012) Secreted human glycyl-tRNA synthetase implicated in defense against ERK-activated tumorigenesis. Proc Natl Acad Sci U S A 109:40–07
Brosius FC, Khoury CC, Buller CL, Chen S (2010) Abnormalities in signalling pathways in diabetic nephropathy. Expert Rev Endocrinol Metab 5:51–64
Sinuani I, Beberashvili I, Averbukh Z, Cohn M, Gitelman I, Weissgarten J (2010) Mesangial cells initiate compensatory tubular cell hypertrophy. Am J Nephrol 31:326–31
Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M (2006) Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol 17:1604–14
Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, Haskó G, Pacher P (2009) Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther 328:708–14
Seifrtová M, Havelek R, Cmielová J, Jiroutová A, Soukup T, Brůčková L, Mokrý J, English D, Rezáčová M (2012) The response of human ectomesenchymal dental pulp stem cells to cisplatin treatment. Int Endod J 45:401–12
Cooper JA, Sefton BM, Hunter T (1984) Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol 4:30–37
Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD (1991) ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65:663–75
Dérijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025–37
Dérijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ (1995) Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682–85
Zhuang S, Schnellmann RG (2006) A death-promoting role for extracellular signal regulated kinase. J Pharmacol Exp Ther 319:991–1007
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–83
Basu A, Tu H (2005) Activation of ERK during DNA damage-induced apoptosis involves protein kinase Cdelta. Biochem Biophys Res Commun 334:1068–73
Wang X, Govind S, Sajankila SP, Mi L, Roy R, Chung FL (2011) Phenethyl isothiocyanate sensitizes human cervical cancer cells to apoptosis induced by cisplatin. Mol Nutr Food Res 55:1572–81
Xu Y, Liu L, Qiu X, Liu Z, Li H, Li Z, Luo W, Wang E (2012) CCL21/CCR7 prevents apoptosis via the ERK pathway in human non-small cell lung cancer cells. PLoS One 7:33262
Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S (2002) Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem 277:29803–09
Tomiyama A, Tachibana K, Suzuki K, Seino S, Sunayama J, Matsuda KI, Sato A, Matsumoto Y, Nomiya T, Nemoto K, Yamashita H, Kayama T, Ando K, Kitanaka C (2010) MEK-ERK dependent multiple caspase activation by mitochondridal proapoptotic Bcl 2family proteins is essential for heavy ion irradiation induced glioma. cell-death 1:60
Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma WY, Dong Z, Pike HM, Brown RE, Reed JC (2005) Calcium-activated RAF/MEK/ERK signalling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell 16:4437–53
Zhou QM, Wang S, Zhang H, Lu YY, Wang XF, Motoo Y, Su SB (2009) The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin 30:1648–58
Ma FY, Flanc RS, Tesch GH, Han Y, Atkins RC, Bennett BL, Friedman GC, Fan JH, Nikolic-Paterson DJ (2007) A pathogenic role for c-Jun amino-terminal kinase signalling in renal fibrosis and tubular cell apoptosis. J Am Soc Nephrol 18:472–84
Morrison DK, Davis RJ (2003) Regulation of MAP kinase signalling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 19:91–118
Dickinson RJ, Keyse SM (2006) Diverse physiological functions for dual specificity MAP kinase phosphatases. J Cell Sci 119:4607–15
Gerits N, Kostenko S, Moens U (2007) In vivo functions of mitogen-activated protein kinases: conclusions from knock-in and knock-out mice. Transgenic Res 16:281–314
Ma FY, Liu J, Nikolic-Paterson DJ (2009) The role of stress-activated protein kinase signalling in renal pathophysiology. Braz J Med Biol Res 42:29–37
Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B (1996) Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 15:2760–70
De Borst MH, Prakash J, Melenhorst WB, van den Heuvel MC, Kok RJ, Navis G, van Goor H (2007) Glomerular and tubular induction of the transcription factor c-Jun in human renal disease. J Pathol 213:219–28
Kyriakis JM, Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81:807–69
Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ (2004) The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol 15:370–79
Flanc RS, Ma FY, Tesch GH, Han Y, Atkins RC, Bennett BL, Friedman GC, Fan JH, Nikolic-Paterson DJ (2007) A pathogenic role for JNK signalling in experimental anti-GBM glomerulonephritis. Kidney Int 7:698–708
Nguyen HT, Hsieh MH, Gaborro A, Tinloy B, Phillips C, Adam RM (2006) JNK/SAPK and p38 SAPK-2 mediate mechanical stretch-induced apoptosis via caspase-3 and −9 in NRK- 52E renal epithelial cells. Nephron Exp Nephrol 102:49–61
Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Di Padova F, Ulevitch RJ, Han J (1997) Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem 272:30122–28
Ma FY, Tesch GH, Flavell RA, Davis RJ, Nikolic-Paterson DJ (2007) MKK3-p38 signalling promotes apoptosis and the early inflammatory response in the obstructed mouse kidney. Am J Physiol 293:1556–63
Ma FY, Tesch GH, Ozols E, Xie M, Schneider MD, Nikolic-Paterson DJ (2011) TGF-β1-activated kinase-1 regulates inflammation and fibrosis in the obstructed kidney. Am J Physiol Renal Physiol 300:1410–21
Adhikary L, Chow F, Nikolic-Paterson DJ, Stambe C, Dowling J, Atkins RC, Tesch GH (2004) Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia 47:1210–22
Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Hara A, Yamahana J, Okumura T, Takasawa K, Takeda S, Yoshimura M, Kida H, Yokoyama H (2005) Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am J Kidney Dis 45:54–65
Lim AK, Tesch GH (2012) Inflammation in diabetic nephropathy. Mediators Inflamm 2012:146154. doi:10.1155/2012/146154
Ramesh G, Reeves WB (2005) p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol 289:166–74
Jiao JW, Wen F (2011) Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Oncol Rep 25:781–88
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB (2010) Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2:2490–518
Mandic A, Hansson J, Linder S, Shoshan MC (2003) Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signalling. J Biol Chem 278:9100–06
Cullen KJ, Yang Z, Schumaker L, Guo Z (2007) Mitochondria as a critical target of the chemotheraputic agent cisplatin in head and neck cancer. J Bioenerg Biomembr 39:43–50
Francescato HD, Costa RS, Júnior FB, Coimbra TM (2007) Effect of JNK inhibition on cisplatin-induced renal damage. Nephrol Dial Transplant 22:2138–48
Bassett EA, Wang W, Rastinejad F, El-Deiry WS (2008) Structural and functional basis for therapeutic modulation of p53 signalling. Clin Cancer Res 14:6376–86
Clark JS, Faisal A, Baliga R, Nagamine Y, Arany I (2010) Cisplatin induces apoptosis through the ERK-p66shc pathway in renal proximal tubule cells. Cancer Lett 297:165–70
Kim YK, Choi TR, Kwon CH, Kim JH, Woo JS, Jung JS (2003) Beneficial effect of pentoxifylline on cisplatin-induced acute renal failure in abbits. Ren Fail 25:909–22
Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara K, Hirakata H, Iida M (2003) Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int 63:72–82
Chauhan P, Sodhi A, Shrivastava A (2009) Cisplatin primes murine peritoneal macrophages for enhanced expression of nitric oxide, proinflammatory cytokines, TLRs, transcription factors and activation of MAP kinases upon co-incubation with L929 cells. Immunobiology 214:197–209
Dong G, Luo J, Kumar V, Dong Z (2010) Inhibitors of histone deacetylases suppress cisplatin-induced p53 activation and apoptosis in renal tubular cells. Am J Physiol Renal Physiol 298:293–300
Cummings BS, Schnellmann RG (2002) Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302:8–17
Jiang M, Yi X, Hsu S, Wang CY, Dong Z (2004) Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287:1140–47
Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP (2005) p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J Biol Chem 280:31230–39
Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z (2006) Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25:4056–66
Jing M, Pabla N, Murph RF, Yang T, Yin XM, Degenhardt K, White E, Dang Z (2007) Nutin-3 protects kidney cells during cisplatin therephy by supressing Bax/Bak activation. J Biol chem 282:2636–45
Jiang M, Wei Q, Pabla N, Dong G, Wang CY, Yang T, Smith SB, Dong Z (2007) Effects of hydroxyl radical scavenging on cisplatin-induced p53 activation, tubular cell apoptosis andnephrotoxicity. Biochem Pharmacol 73:1499–510
Megyesi J, Safirstein RL, Price PM (1998) Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest 101:777–82
Manfredi JJ (2003) p53 and apoptosis: it’s not just in the nucleus anymore. Mol Cell 11:552–54
Bhatt K, Zhou L, Mi QS, Huang S, She JX, Dong Z (2010) MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol Med 16:409–16
Safirstein R, Miller P, Guttenplan JB (1984) Uptake and metabolism of cisplatin by rat kidney. Kidney Int 25:753–58
Hanigan MH, Devarajan P (2003) Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther 1:47–61
Xiao T, Choudhary S, Zhang W, Ansari NH, Salahudeen A (2003) Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells. J Toxicol Environ Health A 66:469–79
Price PM, Safirstein RL, Megyesi J (2004) Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am J Physiol Renal Physiol 286:378–84
Vickers AE, Rose K, Fisher R, Saulnier M, Sahota P, Bentley P (2004) Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol Pathol 32:577–90
May P, May E (1999) Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 18:7621–36
Vousden KH, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604
Oren M (2003) Decision making by p53: life, death and cancer. Cell Death Differ 10:431–42
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B (2000) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7:673–82
Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L (2003) PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A 100:1931–36
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP (2003) Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4:321–28
Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV (1998) In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int 53:394–401
Rao M, Kumar MM, Rao MA (1999) In vitro and in vivo effects of phenolic antioxidants against cisplatin-induced nephrotoxicity. J Biochem 125:383–90
Tsuruya K, Yotsueda H, Ikeda H, Taniguchi M, Masutani K, Hayashida H, Hirakata H, Iida M (2008) Involvement of p53-transactivated Puma in cisplatin induced renal tubular cell death. Life Sci 83:550–56
Dumont P, Leu JI, Della Pietra AC 3rd, George DL, Murphy M (2003) The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet 33:357–65
Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM (2003) p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11:577–90
Erster S, Mihara M, Kim RH, Petrenko O, Moll UM (2004) In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol 24:6728–41
Leu JI, Dumont P, Hafey M, Murphy ME, George DL (2004) Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol 6:443–50
Gupta S, Radha V, Furukawa Y, Swarup G (2001) Direct transcriptional activation of human caspase-1 by tumor suppressor p53. J Biol Chem 276:10585–88
MacLachlan TK, El-Deiry WS (2002) Apoptotic threshold is lowered by p53 transactivation of caspase-6. Proc Natl Acad Sci U S A 99:9492–27
Rikhof B, Corn PG, El-Deiry WS (2003) Caspase 10 levels are increased following DNA damage in a p53-dependent manner. Cancer Biol Ther 2:707–12
Joshi B, Rastogi S, Morris M, Carastro LM, DeCook C, Seto E, Chellappan SP (2007) Differential regulation of human YY1 and caspase 7 promoters by prohibitin through E2F1 and p53 binding sites. Biochem J 401:155–66
Yang C, Kaushal V, Haun RS, Seth R, Shah SV, Kaushal GP (2008) Transcriptional activation of caspase-6 and −7 genes by cisplatin-induced p53 and its functional significance in cisplatin nephrotoxicity. Cell Death Differ 15:530–544
Megyesi J, Udvarhelyi N, Safirstein RL, Price PM (1996) The p53-independent activation of transcription of p21 WAF1/CIP1/SDI1 after acute renal failure. Am J Physiol 271:1211–16
Yu F, Megyesi J, Safirstein RL, Price PM (2005) Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol 289:514–20
Price PM, Yu F, Kaldis P, Aleem E, Nowak G, Safirstein RL, Megyesi J (2006) Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J Am Soc Nephrol 17:2434–42
Nowak G, Price PM, Schnellmann RG (2003) Lack of a functional p21WAF1/CIP1 gene accelerates caspase-independent apoptosis induced by cisplatin in renal cells. Am J Physiol Renal Physiol 285:440–50
Zhou H, Fujigaki Y, Kato A, Miyaji T, Yasuda H, Tsuji T, Yamamoto T, Yonemura K, Hishida A (2006) Inhibition of p21 modifies the response of cortical proximal tubules to cisplatin in rats. Am J Physiol Renal Physiol 291:225–35
Liu WS, Heckman CA (1998) The sevenfold way of PKC regulation. Cell Signal 10:529–42
Kikkawa U, Matsuzaki H, Yamamoto T (2002) Protein kinase C delta (PKC delta): activation mechanisms and functions. J Biochem 132:831–39
Parker PJ, Murray-Rust J (2004) PKC at a glance. J Cell Sci 117:131–32
Jackson DN, Foster DA (2004) The enigmatic protein kinase Cdelta: complex roles in cell proliferation and survival. FASEB J 18:627–36
DeVries-Seimon TA, Ohm AM, Humphries MJ, Reyland ME (2007) Induction of apoptosis is driven by nuclear retention of protein kinase C delta. J Biol Chem 282:22307–14
Humphries MJ, Ohm AM, Schaack J, Adwan TS, Reyland ME (2008) Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene 27:3045–53
Matsushima H, Yonemura K, Ohishi K, Hishida A (1998) The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med 131:518–26
Davis CA, Nick HS, Agarwal A (2001) Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol 12:2683–90
Chirino YI, Hernández-Pando R, Pedraza-Chaverrí J (2004) Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin induced nephrotoxicity in rats. BMC Pharmacol 4:20
Chirino YI, Trujillo J, Sánchez-González DJ, Martínez-Martínez CM, Cruz C, Bobadilla NA, Pedraza-Chaverri J (2008) Selective iNOS inhibition reduces renal damage induced by cisplatin. Toxicol Lett 176:48–57
Ries F, Klastersky J (1986) Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis 8:368–79
Racz I, Tory K, Gallyas F Jr, Berente Z, Osz E, Jaszlits L, Bernath S, Sumegi B, Rabloczky G, Literati-Nagy P (2002) BGP-15 - a novel poly(ADP-ribose) polymerase inhibitor-protects against nephrotoxicity of cisplatin without compromising its antitumor activity. Biochem Pharmacol 63:1099–111
Mukhopadhyay P, Pan H, Rajesh M, Bátkai S, Patel V, Harvey-White J, Mukhopadhyay B, Haskó G, Gao B, Mackie K, Pacher P (2010) CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol 160:657–68
Baud L, Ardaillou R (1986) Reactive oxygen species: production and role in the kidney. Am J Physiol 251:765–76
Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF (1997) Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther 280:638–49
Satoh M, Kashihara N, Fujimoto S, Horike H, Tokura T, Namikoshi T, Sasaki T, Makino H (2003) A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther 305:1183–90
Taylor RW, Turnbull DM (2005) Mitochondrial DNA mutations in human disease. Nat Rev Genet 6:389–402
Park MS, De Leon M, Devarajan P (2002) Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol 13:858–65
Schreck R, Rieber P, Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 10:2247–58
Baeuerle PA, Henkel T (1994) Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12:141–79
Barnes PJ, Karin M (1997) nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–71
Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA (2001) Interleukin-10 inhibits ischemic and cisplatin induced acute renal injury. Kidney Int 60:2118–28
Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP, Jang YB, Lee JE, Jang KY, Park SK (2006) Rosiglitazone ameliorates cisplatin-induced renal injury in mice. Nephrol Dial Transplant 21:2096–105
Sieber S, Lange N, Kollmorgen G, Erhardt A, Quaas A, Gontarewicz A, Sass G, Tiegs G, Kreienkamp HJ (2012) Sharpin contributes to TNFα dependent NFκB activation and anti-apoptotic signalling in hepatocytes. PLoS One 7:29993
Greene EL, Paller MS (1991) Oxygen free radicals in acute renal failure. Miner Electrolyte Metab 17:124–32
Paller MS, Neumann TV (1991) Reactive oxygen species and rat renal epithelial cells during hypoxia and reoxygenation. Kidney Int 40:1041–9
Paller MS (1992) Free radical-mediated postischemic injury in renal transplantation. Ren Fail 14:257–60
Baek SM, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK (2003) Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J Lab Clin Med 142:178–86
Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J (2002) Redox control of cell death. Antioxid Redox Signal 4:405–14
Torres M, Forman HJ (2003) Redox signalling and the MAP kinase pathways. Biofactors 17:287–96
Ishikawa Y, Kitamura M (2000) Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int 58:1078–87
Park C, So HS, Kim SJ, Youn MJ, Moon BS, Shin SH, Lee I, Moon SK, Park R (2006) Samul extract protects against the H2O2-induced apoptosis of H9c2 cardiomyoblasts via activation of extracellular regulated kinases (Erk) 1/2. Am J Chin Med 34:695–706
Alscher DM, Braun N, Biegger D, Stuelten C, Gawronski K, Mürdter TE, Kuhlmann U, Fritz P (2005) Induction of metallothionein in proximal tubular cells by zinc and its potential as an endogenous antioxidant. Kidney Blood Press Res 28:127–33
Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, Jin L, Xiao J, Xie R, Rane M, Li X, Cai L (2010) Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem 21:237–46
Abdelrahman AM, Al Salam S, AlMahruqi AS, IS A h, Mansour MA, Ali BH (2010) N-acetylcysteine improves renal hemodynamics in rats with cisplatin-induced nephrotoxicity. J Appl Toxicol 30:15–21
Shalby AB, Assaf N, Ahmed HH (2011) Possible mechanisms for N-acetyl cysteine and taurine in ameliorating acute renal failure induced by cisplatin in rats. Toxicol Mech Methods 21:538–46
Patel Manali B, Deshpande S, Shah G (2001) Evaluation of efficacy of vitamin E and N-acetyl cysteine in gentamicin-induced nephrotoxicity in rats. Ren Fail 33:341–7
McWhinney SR, Goldberg RM, McLeod HL (2009) Platinum Neurotoxicity Pharmacogenetics. Mol Cancer Ther 8:10–6
Rybak LP, Mukherjea D, Jajoo S, Ramkumar V (2009) Cisplatin Ototoxicity and Protection: Clinical and Experimental. Tohoku J Exp Med 219:177–186
Menon A, Krishnan Nair CK (2013) Ayurvedic formulations ameliorate cisplatin- induced nephrotoxicity: Preclinical studies on Brahma Rasayana and Chyavanapras. J Cancer Res Ther 9:230–4
Cayır K, Karadeniz A, Simşek N, Yıldırım S, Karakuş E, Kara A, Akkoyun HT, Sengül E (2011) Pomegranate seed extract attenuates chemotherapy-induced acute nephrotoxicity and hepatotoxicity in rats. J Med Food 14:1254–62
Sahu BD, Rentam KK, Putcha UK, Kuncha M, Vegi GM, Sistla R (2011) Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem Toxicol 49:3090–7
Yapar K, Cavuşoğlu K, Oruç E, Yalçin E (2009) Protective effect of royal jelly and green tea extracts effect against cisplatin-induced nephrotoxicity in mice: a comparative study. J Med Food 12:1136–42
Sahu BD, Kuncha M, Sindhura GJ, Sistla R (2013) Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 20:453–60
Khan MA, Liu J, Kumar G, Skapek SX, Falck JR, Imig JD (2013) Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2013 Apr 19. [Epub ahead of print]
Acknowledgments
We express our gratefulness to Dr. Pitchai Balakumar forhis expertise, suggestions and review; gratitude is extended to Dr. Rajendar Singh, Chairman, and Shri Om Parkash, Director, Mr. Sanjeev Kalra, Administrator, Rajendra Institute of Technology and Sciences, Sirsa, India, for their inspiration and constant support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaiman, S., Sharma, A.K., Singh, K. et al. Signalling mechanisms involved in renal pathological changes during cisplatin-induced nephropathy. Eur J Clin Pharmacol 69, 1863–1874 (2013). https://doi.org/10.1007/s00228-013-1568-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1568-7