Abstract
Purpose
Pre-clinical experiments have shown that almorexant, a dual orexin receptor antagonist, is able to inhibit cytochrome P450 3A4 (CYP3A4). Therefore, a study was conducted to investigate the effects of multiple-dose almorexant on the pharmacokinetics of midazolam and simvastatin, two CYP3A4 model substrates.
Methods
Fourteen healthy male subjects were enrolled in an open-label, randomized, two-way crossover study. Treatment period A consisted of a single oral dose of 2 mg midazolam on day 1 and 40 mg simvastatin on day 3. In treatment period B, subjects received 200 mg almorexant once daily for 9 days together with a single oral dose of midazolam on day 7 and simvastatin on day 9.
Results
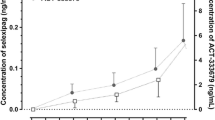
Concomitant administration of midazolam with almorexant at steady-state levels, achieved within 4–5 days, resulted in an increase of 1.2-fold [90 % confidence interval (CI) 1.0–1.4], 1.4-fold (90 % CI 1.2–1.6), and 1.3-fold (90 % CI 1.2–1.4) in the maximum plasma concentration (Cmax), area under the concentration–time curve from time 0 to infinity (AUC0-∞), and terminal half-life (t1/2), respectively, of midazolam; the time to peak plasma concentration (tmax) was unchanged. Whereas Cmax and tmax were not influenced by almorexant, the AUC0-∞ of hydroxy-midazolam increased by 1.2-fold (90 % CI 1.1–1.4) and the t1/2 by 1.3-fold (90 % CI 1.0–1.5). Concomitant administration of simvastatin with almorexant at steady-state resulted in an increase of 2.7-fold (90 % CI 2.0–3.7) and 3.4-fold (90 % CI 2.6–4.4) in Cmax and AUC0-∞, respectively, for simvastatin; the t1/2 and tmax were unchanged. The Cmax and AUC0-∞ of hydroxyacid simvastatin both increased by 2.8-fold, with 90 % CIs of 2.3–3.5 and 2.2–3.5, respectively; the tmax increased by 2 h and the t1/2 was unchanged. The urinary 6-β-hydroxycortisol/cortisol ratio was unaffected by almorexant.
Conclusions
Our results suggest that the observed interaction was caused by the inhibition of CYP3A4 activity, most probably at the gut level.




Similar content being viewed by others
References
Sakurai T (2007) The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8(3):171–181
Tsujino N, Sakurai T (2009) Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev 61(2):162–176
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92(4):573–585
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95(1):322–327
Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG (2006) Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol Rev 58(1):46–57
Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F (2007) Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 13(2):150–155
Hoever P, de Haas S, Winkler J, Schoemaker RC, Chiossi E, van Gerven J, Dingemanse J (2010) Orexin receptor antagonism, a new sleep-promoting paradigm: an ascending single-dose study with almorexant. Clin Pharmacol Ther 87(5):593–600
Hoever P, de Haas S, Winkler J, Schoemaker RC, Chiossi E, van Gerven J, Dingemanse J Orexin receptor antagonism, a new sleep-promoting paradigm: an ascending single-dose study with almorexant. Clin Pharmacol Ther 87 (5):593–600
Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, Garson SL, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ (2011) Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet 25(1–2):52–61
Winrow CJ, Gotter AL, Cox CD, Tannenbaum PL, Garson SL, Doran SM, Breslin MJ, Schreier JD, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ (2011) Pharmacological characterization of MK-6096—a dual orexin receptor antagonist for insomnia. Neuropharmacology 62:978–987
Bettica P, Nucci G, Pyke C, Squassante L, Zamuner S, Ratti E, Gomeni R, Alexander R (2012) Phase I studies on the safety, tolerability, pharmacokinetics and pharmacodynamics of SB-649868, a novel dual orexin receptor antagonist. J Psychopharmacol 26(8):1058–1070
Zhou SF (2008) Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab 9(4):310–322
Ogu CC, Maxa JL (2000) Drug interactions due to cytochrome P450. Proc Bayl Univ Med Cent 13(4):421–423
Hesse LM, von Moltke LL, Greenblatt DJ (2003) Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs 17(7):513–532
U.S. Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) (2012( Draft guidance for industry, drug interaction studies-study design, data analysis, and implications for dosing and labeling. U.S. Department of Health and Human Services, FDA, CDER, CBER, Washington D.C.
Galteau MM, Shamsa F (2003) Urinary 6beta-hydroxycortisol: a validated test for evaluating drug induction or drug inhibition mediated through CYP3A in humans and in animals. Eur J Clin Pharmacol 59(10):713–733
Shimizu M, Uno T, Tamura HO, Kanazawa H, Murakami I, Sugawara K, Tateishi T (2007) A developed determination of midazolam and 1′-hydroxymidazolam in plasma by liquid chromatography-mass spectrometry: application of human pharmacokinetic study for measurement of CYP3A activity. J Chromatogr B Analyt Technol Biomed Life Sci 847(2):275–281
Dingemanse J, Schaarschmidt D, van Giersbergen PL (2003) Investigation of the mutual pharmacokinetic interactions between bosentan, a dual endothelin receptor antagonist, and simvastatin. Clin Pharmacokinet 42(3):293–301
Molden E, Skovlund E, Braathen P (2008) Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors. Drug Saf 31(7):587–596
Rowan C, Brinker AD, Nourjah P, Chang J, Mosholder A, Barrett JS, Avigan M (2009) Rhabdomyolysis reports show interaction between simvastatin and CYP3A4 inhibitors. Pharmacoepidemiol Drug Saf 18(4):301–309
Bottorff MB (2006) Statin safety and drug interactions: clinical implications. Am J Cardiol 97(8A):27C–31C
Vickers S, Duncan CA, Chen IW, Rosegay A, Duggan DE (1990) Metabolic disposition studies on simvastatin, a cholesterol-lowering prodrug. Drug Metab Dispos 18(2):138–145
Vickers S, Duncan CA, Vyas KP, Kari PH, Arison B, Prakash SR, Ramjit HG, Pitzenberger SM, Stokker G, Duggan DE (1990) In vitro and in vivo biotransformation of simvastatin, an inhibitor of HMG CoA reductase. Drug Metab Dispos 18(4):476–483
Neuvonen PJ, Niemi M, Backman JT (2006) Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther 80(6):565–581
Shitara Y, Sugiyama Y (2006) Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug–drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 112(1):71–105
Prueksaritanont T, Gorham LM, Ma B, Liu L, Yu X, Zhao JJ, Slaughter DE, Arison BH, Vyas KP (1997) In vitro metabolism of simvastatin in humans [SBT] identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos 25(10):1191–1199
Prueksaritanont T, Ma B, Yu N (2003) The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol 56(1):120–124
Hoever P, de Haas SL, Dorffner G, Chiossi E, van Gerven JM, Dingemanse J (2012) Orexin receptor antagonism: an ascending multiple-dose study with almorexant. J Psychopharmacol 26(8):1071–1080
Kupferschmidt HH, Ha HR, Ziegler WH, Meier PJ, Krahenbuhl S (1995) Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther 58(1):20–28
Hanley MJ, Cancalon P, Widmer WW, Greenblatt DJ (2011) The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol 7(3):267–286
Lilja JJ, Neuvonen M, Neuvonen PJ (2004) Effects of regular consumption of grapefruit juice on the pharmacokinetics of simvastatin. Br J Clin Pharmacol 58(1):56–60
Tsunoda SM, Velez RL, von Moltke LL, Greenblatt DJ (1999) Differentiation of intestinal and hepatic cytochrome P450 3A activity with use of midazolam as an in vivo probe: effect of ketoconazole. Clin Pharmacol Ther 66(5):461–471
Holtzman CW, Wiggins BS, Spinler SA (2006) Role of P-glycoprotein in statin drug interactions. Pharmacotherapy 26(11):1601–1607
Kalliokoski A, Niemi M (2009) Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 158(3):693–705
Chen C, Mireles RJ, Campbell SD, Lin J, Mills JB, Xu JJ, Smolarek TA (2005) Differential interaction of 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos 33(4):537–546
Disclosure of conflict of interest
This study was sponsored by Actelion Pharmaceuticals Ltd. Matthias Hoch and Jasper Dingemanse are full-time employees of Actelion Pharmaceuticals Ltd. Petra Hoever was a full-time employee of Actelion Pharmaceuticals Ltd at the time the study was conducted and the data analyzed. Federica Alessi was a full-time employee of Actelion Pharmaceuticals Italia Srl, Biostatistics at time the study was conducted and the data analyzed. PHAROS GmbH received funding from Actelion Pharmaceuticals Ltd. Rudolf Theodor was a full-time employee of PHAROS GmbH at the time the study was conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoch, M., Hoever, P., Alessi, F. et al. Pharmacokinetic interactions of almorexant with midazolam and simvastatin, two CYP3A4 model substrates, in healthy male subjects. Eur J Clin Pharmacol 69, 523–532 (2013). https://doi.org/10.1007/s00228-012-1403-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1403-6