Abstract
Purpose
The purpose of this study was to develop a population pharmacokinetic (PPK) model of glimepiride and to investigate the influence of genetic polymorphisms in CYP2C9 on the PPK of glimepiride in healthy Korean subjects.
Methods
Serum data after a single oral dose of 2 mg of glimepiride in 177 healthy male Korean subjects (CYP2C9*1*1: 163 subjects, *1/*3: 14 subjects) were used. We estimated the PPK of glimepiride using a nonlinear mixed effects modeling (NONMEM) method and explored the possible influence of genetic polymorphisms in CYP2C9 on the PPK of glimepiride.
Results
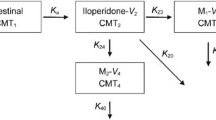
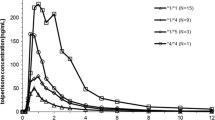
The disposition of glimepiride was best described with a two-compartment model with a Weibull-type absorption and first-order elimination. The visual predictive check indicated that the pharmacokinetic profile of glimepiride was adequately described by the proposed PPK model. The CYP2C9 genotypes as covariate significantly (P < 0.001) influenced the apparent oral clearance (CL/F) of glimepiride. The estimated CL/F of glimepiride was higher (1.60-fold) in CYP2C9*1/*1 subjects than in CYP2C9*1/*3 subjects.
Conclusions
This study indicates that genetic polymorphisms of CYP2C9 influence the substantial interindividual variability in the disposition of glimepiride, and these polymorphisms may affect the clinical response to glimepiride therapy.




Similar content being viewed by others
References
Kramer W, Muller G, Geisen K (1996) Characterization of the molecular mode of action of the sulfonylurea, glimepiride, at beta-cells. Horm Metab Res 28(9):464–468. doi:10.1055/s-2007-979838
Frick A, Moller H, Wirbitzki E (1998) Biopharmaceutical characterization of oral immediate release drug products. In vitro/in vivo comparison of phenoxymethylpenicillin potassium, glimepiride and levofloxacin. Eur J Pharm Biopharm 46(3):305–311
Badian M, Korn A, Lehr KH, Malerczyk V, Waldhausl W (1994) Absolute bioavailability of glimepiride (Amaryl) after oral administration. Drug Metabol Drug Interact 11(4):331–339
Benet LZ (2010) Predicting drug disposition via application of a Biopharmaceutics Drug Disposition Classification System. Basic Clin Pharmacol Toxicol 106(3):162–167. doi:10.1111/j.1742-7843.2009.00498.x
Malerczyk V, Badian M, Korn A, Lehr KH, Waldhausl W (1994) Dose linearity assessment of glimepiride (Amaryl) tablets in healthy volunteers. Drug Metabol Drug Interact 11(4):341–357
Langtry HD, Balfour JA (1998) Glimepiride. A review of its use in the management of type 2 diabetes mellitus. Drugs 55(4):563–584
Yamazaki H, Tabata S (1993) Sex difference in pharmacokinetics of the novel sulfonylurea antidiabetic glimepiride in rats. Arzneimittelforschung 43(12):1317–1321
Niemi M, Cascorbi I, Timm R, Kroemer HK, Neuvonen PJ, Kivisto KT (2002) Glyburide and glimepiride pharmacokinetics in subjects with different CYP2C9 genotypes. Clin Pharmacol Ther 72(3):326–332. doi:10.1067/mcp.2002.127495
Suzuki K, Yanagawa T, Shibasaki T, Kaniwa N, Hasegawa R, Tohkin M (2006) Effect of CYP2C9 genetic polymorphisms on the efficacy and pharmacokinetics of glimepiride in subjects with type 2 diabetes. Diabetes Res Clin Pract 72(2):148–154. doi:10.1016/j.diabres.2005.09.019
Daly AK (2003) Pharmacogenetics of the major polymorphic metabolizing enzymes. Fundam Clin Pharmacol 17(1):27–41
LLerena A, Dorado P, O’Kirwan F, Jepson R, Licinio J, Wong ML (2004) Lower frequency of CYP2C9*2 in Mexican-Americans compared to Spaniards. Pharmacogenomics J 4(6):403–406. doi:10.1038/sj.tpj.65002786500278
Xie HG, Prasad HC, Kim RB, Stein CM (2002) CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev 54(10):1257–1270
Zainuddin Z, Teh LK, Suhaimi AW, Salleh MZ, Ismail R (2003) A simple method for the detection of CYP2C9 polymorphisms: nested allele-specific multiplex polymerase chain reaction. Clin Chim Acta 336(1–2):97–102
Shon JH, Yoon YR, Kim KA, Lim YC, Lee KJ, Park JY, Cha IJ, Flockhart DA, Shin JG (2002) Effects of CYP2C19 and CYP2C9 genetic polymorphisms on the disposition of and blood glucose lowering response to tolbutamide in humans. Pharmacogenetics 12(2):111–119
Miners JO, Birkett DJ (1998) Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45(6):525–538
Lehr KH, Damm P (1990) Simultaneous determination of the sulphonylurea glimepiride and its metabolites in human serum and urine by high-performance liquid chromatography after pre-column derivatization. J Chromatogr 526(2):497–505
Beal S, Sheiner L (1992) NONMEM user’s guide, part I. University of California at San Francisco, San Francisco
Bressolle F, Gomeni R, Alric R, Royer-Morrot MJ, Necciari J (1994) A double Weibull input function describes the complex absorption of sustained-release oral sodium valproate. J Pharm Sci 83(10):1461–1464
Rousseau A, Leger F, Le Meur Y, Saint-Marcoux F, Paintaud G, Buchler M, Marquet P (2004) Population pharmacokinetic modeling of oral cyclosporin using NONMEM: comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Ther Drug Monit 26(1):23–30
Yun HY, Park HC, Kang W, Kwon KI (2006) Pharmacokinetic and pharmacodynamic modelling of the effects of glimepiride on insulin secretion and glucose lowering in healthy humans. J Clin Pharm Ther 31(5):469–476. doi:10.1111/j.1365-2710.2006.00766.x
Ludden TM, Beal SL, Sheiner LB (1994) Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm 22(5):431–445
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm 20(5):511–528
Jonsson EN, Karlsson MO (1999) Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58(1):51–64
Hooker AC, Staatz CE, Karlsson MO (2007) Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24(12):2187–2197. doi:10.1007/s11095-007-9361-x
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79(3):241–257. doi:10.1016/j.cmpb.2005.04.005
Savic RM, Jonker DM, Kerbusch T, Karlsson MO (2007) Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34(5):711–726. doi:10.1007/s10928-007-9066-0
Weiss M (1996) A novel extravascular input function for the assessment of drug absorption in bioavailability studies. Pharm Res 13(10):1547–1553
Kirchheiner J, Bauer S, Meineke I, Rohde W, Prang V, Meisel C, Roots I, Brockmoller J (2002) Impact of CYP2C9 and CYP2C19 polymorphisms on tolbutamide kinetics and the insulin and glucose response in healthy volunteers. Pharmacogenetics 12(2):101–109
Lee CR, Goldstein JA, Pieper JA (2002) Cytochrome P450 2 C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 12(3):251–263
Yoon YR, Shon JH, Kim MK, Lim YC, Lee HR, Park JY, Cha IJ, Shin JG (2001) Frequency of cytochrome P450 2 C9 mutant alleles in a Korean population. Br J Clin Pharmacol 51(3):277–280
Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, Park YS, Lee SY (2005) Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol 60(4):418–422. doi:10.1111/j.1365-2125.2005.02448.x
Myrand SP, Sekiguchi K, Man MZ, Lin X, Tzeng RY, Teng CH, Hee B, Garrett M, Kikkawa H, Lin CY, Eddy SM, Dostalik J, Mount J, Azuma J, Fujio Y, Jang IJ, Shin SG, Bleavins MR, Williams JA, Paulauskis JD, Wilner KD (2008) Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther 84(3):347–361. doi:10.1038/sj.clpt.6100482
Wang R, Chen K, Wen SY, Li J, Wang SQ (2005) Pharmacokinetics of glimepiride and cytochrome P450 2 C9 genetic polymorphisms. Clin Pharmacol Ther 78(1):90–92. doi:10.1016/j.clpt.2005.03.008
Kirchheiner J, Roots I, Goldammer M, Rosenkranz B, Brockmoller J (2005) Effect of genetic polymorphisms in cytochrome p450 (CYP) 2 C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet 44(12):1209–1225
Matsuki M, Matsuda M, Kohara K, Shimoda M, Kanda Y, Tawaramoto K, Shigetoh M, Kawasaki F, Kotani K, Kaku K (2007) Pharmacokinetics and pharmacodynamics of glimepiride in type 2 diabetic patients: compared effects of once- versus twice-daily dosing. Endocr J 54(4):571–576
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A070001).
Competing interests
None to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hee-Doo Yoo and Mi-Suk Kim contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yoo, HD., Kim, MS., Cho, HY. et al. Population pharmacokinetic analysis of glimepiride with CYP2C9 genetic polymorphism in healthy Korean subjects. Eur J Clin Pharmacol 67, 889–898 (2011). https://doi.org/10.1007/s00228-011-1035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1035-2