Abstract
Purpose
To examine the pharmacokinetics of amikacin and its pharmacokinetic pharmacodynamic (PKPD) relationship in neonates. To develop an alternative dosing strategy for amikacin in neonates.
Methods
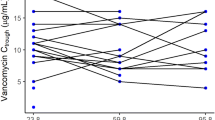
A population PKPD analysis was performed using data collected from 80 neonates with gestational ages from 24 to 41 weeks. The final pharmacokinetic model analysed 358 amikacin concentrations. All neonates were > 72 hours postnatal age. Simulations were performed to develop a new dosing strategy.
Results
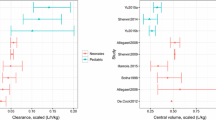
The final covariate model was clearance = 0.23 × (current weight/2)0.691 × (postmenstrual age/40)3.23 and volume of distribution = 0.957 × (current weight/2)0.89. Following the logistic regression analysis of treatment failure, new amikacin target concentrations were estimated and used in development of an alternative dosing strategy.
Conclusion
Simulation of a new dosing regimen yielded the following recommendations: 15 mg/kg at 36-h intervals, 14 mg/kg at 24-h intervals and 15 mg/kg at 24-h intervals for neonates ≤28 weeks, 29–36 weeks and ≥37 weeks postmenstrual age respectively.


Similar content being viewed by others
References
Clapp DW (2006) Developmental regulation of the immune system. Semin Perinatol 30:69–72
Petersen PO, Wells TG, Kearns GL (1991) Amikacin dosing in neonates: evaluation of a dosing chart based on population pharmacokinetic data. Dev Pharmacol Ther 16:203–211
Zaske DE, Strate RG, Kohls PR (1991) Amikacin pharmacokinetics—wide interpatient variation in 98 patients. J Clin Pharmacol 31:158–163
Bristol-Meyers S (2001) Amikin. Bristol-Myers Squibb Pharmaceuticals, Noble Park, Australia
Bleyzac N, Varnier V, Labaune JM, Corvaisier S, Maire P, Jelliffe RW, Putet G, Aulagner G (2001) Population pharmacokinetics of amikacin at birth and interindividual variability in renal maturation. Eur J Clin Pharmacol 57:499–504
Young TE, Mangum B (2002) Neofax, 15 ed. Acorn Publishing, Raleigh
Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12
Langhendries JP, Battisti O, Bertrand JM, Francois A, Kalenga M, Darimont J, Scalais E, Wallemacq P (1998) Adaptation in neonatology of the once-daily concept of aminoglycoside administration: evaluation of a dosing chart for amikacin in an intensive care unit. Biol Neonate 74:351–362
Cookson B, Tripp J, Leung T, Williams JD (1980) Evaluation of amikacin dosage regimes in the low and very low birthweight newborn. Infection 8(Suppl 3):239–242
Ette EL, Williams PJ, Kim YH, Lane JR, Liu MJ, Capparelli EV (2003) Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol 43:610–623
Pagano M, Gauvreau K (1993) Principles of biostatistics. Wadsworth Publishing, Belmont, CA
Kotze A, Bartel PR, Sommers DK (1999) Once versus twice daily amikacin in neonates: prospective study on toxicity. J Paediatr Child Health 35:283–286
Langhendries JP, Battisti O, Bertrand JM, Francois A, Darimont J, Ibrahim S, Tulkens PM, Bernard A, Buchet JP, Scalais E (1993) Once-a-day administration of amikacin in neonates: assessment of nephrotoxicity and ototoxicity. Dev Pharmacol Ther 20:220–230
Botha FJH, van der Bijl P, Seifart HI, Parkin DP (1996) Fluctuation of the volume of distribution of amikacin and its effect on once-daily dosage and clearance in a seriously ill patient. Intensive Care Med 22:443–446
Assael BM, Parini R, Rusconi F, Cavanna G (1982) Influence of intrauterine maturation on the pharmacokinetics of amikacin in the neonatal period. Pediatr Res 16:810–815
Botha JH, du Preez M, Miller R, Adhikari M (1998) Determination of population pharmacokinetic parameters for amikacin in neonates using mixed-effect models. Eur J Clin Pharmacol 53:337–341
Kenyon CF, Knoppert DC, Lee SK, Vandenberghe HM, Chance GW (1990) Amikacin pharmacokinetics and suggested dosage modifications for the preterm infant. Antimicrob Agents Chemother 34:265–268
Berger A, Kretzer V, Gludovatz P, Heinze G, Haiden N, Pollak A (2004) Evaluation of an amikacin loading dose for nosocomial infections in very low birthweight infants. Acta Paediatrica 93:356–360
Savic RM, Karlsson MO (2007) Importance of shrinkage in empirical bayes estimates for diagnostics and estimation: problems and solutions. Abstracts of the Annual Meeting of the Population Approach Group in Europe, p. 16
Allegaert K, Cossey V, Langhendries JP, Naulaers G, Vanhole C, Devlieger H, Van Overmeire B (2004) Effects of co-administration of ibuprofen-lysine on the pharmacokinetics of amikacin in preterm infants during the first days of life. Biol Neonate 86:207–211
Treluyer JM, Merle Y, Tonnelier S, Rey E, Pons G (2002) Nonparametric population pharmacokinetic analysis of amikacin in neonates, infants, and children. Antimicrob Agents Chemother 46:1381–1387
Koren G, James A, Perlman M (1985) Simple method for the estimation of glomerular filtration rate by gentamicin pharmacokinetics during routine drug monitoring in the newborn. Clin Pharmacol Ther 38:680–685
Allegaert K, Anderson BJ, Cossey V, Holford NHG (2005) Limited predictability of amikacin clearance in extreme premature neonates at birth. Br J Clin Pharmacol 61:39–48
Bueva A, Guignard JP (1994) Renal function in preterm neonates. Pediatr Res 36:572–577
Kenyon CF, Knoppert DC, Lee SK, Vandenberghe HM, Chance GW (1990) Amikacin pharmacokinetics and suggested dosage modifications for the preterm infant. Antimicrob Agents Chemother 34:265–268
Lingvall M, Reith DM, Broadbent R (2005) The effect of sepsis upon gentamicin pharmacokinetics in neonates. Br J Clin Pharmacol 59:54–61
Mann HJ, Fuhs DW, Awang R, Ndemo FA, Cerra FB (1987) Altered aminoglycoside pharmacokinetics in critically ill patients with sepsis. Clin Pharm 6:148–153
Padovani EM, Pistolesi C, Fanos V, Messori A, Martini N (1993) Pharmacokinetics of amikacin in neonates. Dev Pharmacol Ther 20:167–173
Besunder JB, Reed MD, Blumer JL (1988) Principles of drug biodisposition in the neonate. A critical evaluation of the pharmacokinetic-pharmacodynamic interface (part ii). Clin Pharmacokinet 14:261–286
van den Anker JN (1996) Pharmacokinetics and renal function in preterm infants. Acta Paediatr 85:1393–1399
Aperia A, Broberger O, Elinder G, Herin P, Zetterstrom R (1981) Postnatal development of renal function in pre-term and full-term infants. Acta Paediatr Scand 70:183–187
Moore RD, Smith CR, Lietman PS (1984) Association of aminoglycosides plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med 77:657–662
Moore RD, Lietman PS, Smith CR (1987) Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99
Noone P, Parsons TM, Pattison JR, Slack RC, Garfield-Davies D, Hughes K (1974) Experience in monitoring gentamicin therapy during treatment of serious gram-negative sepsis. Br Med J 1:477–481
Acknowledgements
The authors would like to acknowledge Professor Stephen Duffull, School of Pharmacy, University of Otago, Dunedin, New Zealand, for his assistance with the PK modelling and the simulations. Catherine Sherwin is supported by a Freemasons Postgraduate Fellowship in Paediatrics and Child Health from the Freemasons of New Zealand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sherwin, C.M.T., Svahn, S., Van Der Linden, A. et al. Individualised dosing of amikacin in neonates: a pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol 65, 705–713 (2009). https://doi.org/10.1007/s00228-009-0637-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0637-4