Abstract
Objective
To systematically investigate the relationships between thiopurine S-methyltransferase (TPMT) polymorphisms and azathioprine-related adverse drug reactions in patients with kidney transplantation.
Methods
Erythrocyte TPMT activity of 150 patients with kidney transplantation and AZA therapy was determined by HPLC. The frequency of four common TPMT mutant alleles, TPMT*2, *3A, *3B, and *3C was determined by allele-specific PCR and PCR-restriction fragment length polymorphism (PCR-RFLP) analysis.
Results
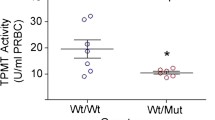
Thirty cases (20%) had stopped azathioprine medication or were on reduced dose due to azathioprine-related side effects. The TPMT activity range of cases who never experienced side effects was 16.63-68.25 U, the mean of the controls was 38.43 ± 11.59 U. The mean value of 12 cases with hematotoxicity was 23.50 ± 10.33 U, much lower than the control mean (P < 0.05). No significant difference between the mean value of 18 cases with hepatotoxicity and the control mean (P > 0.05) was seen. No case with TPMT deficiency was found in all patients studied, and TPMT*2, *3A, and *3B were not detected in any of them. TPMT*3C heterozygous alleles were found in 4.7% (seven cases) of these patients, all seven cases had intermediate TPMT activity, and the mean was 16.75 ± 2.09 U, much lower than other TPMT wild-type patients (P < 0.05). In the seven TPMT*3C patients, four cases experienced side effects (hematotoxicity, n = 2; hepatotoxicity, n = 2).
Conclusions
This study demonstrates that TPMT activity is reduced in patients with TPMT*3C mutation. AZA-induced hematotoxicity is related to the reduced TPMT activity.


Similar content being viewed by others
References
Evans WE (2004) Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy. Ther Drug Monit 26:186–191
Dubinsky ML (2004) Azathioprine, mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, safety. Clin Gastroenterol Hepatol 2:731–743
Lennard L (1998) Clinical implication of thiopurine methyltransferase optimization of drug dosage and potential drug interaction. Ther Drug Monit 20:527–531
Schaeffeler E, Fischer C, Brockmeier D et al (2004) Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics 14:407–417
Schaeffer E, Zanger UM, Eichelbaum M et al (2008) Highly multiplexed genotyping of thiopurine S-methyltransferase variants using MALD-TOF mass spectrometry: reliable genotyping in different ethnic groups. Clin Chem 54:1598–1599
Ujiie S, Sasaki T, Mizygaki M et al (2008) Functional characterization of 23 allelic variants of thiopurine S-methyltransferase gene (TPMT*2-*24). Pharmacogenet Genomics 18:887–893
Mcleod HL, Pritchard SC, Githang’a J et al (1999) Ethnic differences in thiopurine methyltransferase pharmacogenetics: evidence for allele specificity in Caucasian and Kenyan individuals. Pharmcogenetics 9:773–776
Collie-Duguid ES, Pritchard SC, Powrie RH et al (1999) The frequency and distribution of thiopurine methyltransferase allele in Caucasian and Asian populations. Pharmcogenetics 9:37–42
Colombel JF, Ferrari N, Debuysere H et al (2000) Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology 118:1025–1030
Relling MV, Hancock ML, Rivera GK et al (1999) Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 91:2001–2008
Breen DP, Marinaki AM, Arenas M, Hayes PC (2005) Pharmacogenetic association with adverse drug reactions to azathioprine immunosuppressive therapy following liver transplantation. Liver Transplant 11:826–833
Xin HW, Fischer C, Schwab M, Klotz U (2005) Effects of amino salicylates on thiopurine S-methyltransferase activity: an ex vivo study in patients with inflammatory bowel disease. Aliment Pharmacol Ther 21:1105–1109
Kröplin T, Weyer N, Gutsche S, Iven H (1998) Thiopurine S-methyltransferase activity in human erythrocytes: a new HPLC method using 6-thioguanine as substrate. Eur J Clin Pharmacol 54:265–271
Schaeffeler E, Lang T, Zanger UM, Eichelbaum M, Schwab M (2001) High-throughput genotyping of thiopurine S-methyltransferase by denaturing HPLC. Clin Chem 47:548–555
Zhang JP, Zhou SF, Chen X, Huang M (2006) Determination of intra-ethnic differences in the polymorphisms of thiopurine S-methyltransferase in Chinese. Clin Chim Acta 365:337–341
McLeod HL, Siva C (2002) The thiopurine S-methyltransferase gene locus implications for clinical pharmacogenomics. Pharmacogenomics 3:89–98
Krynetski EY, Evans WE (2000) Genetic polymorphism of thiopurine S-methyltransferase: molecular mechanisms and clinical importance. Pharmacology 61:136–146
Huang M, Jiang WQ, Lou YL, Cheng MX (2000) Comparison of thiopurine methyltransferase activity between Chinese and Caucasian populations. Chin J Cancer 19:858–861
Ye Q, Gu L, Zhao J, Liang A, Ye Y (2000) The study on hereditary polymorphism of thiopurine S-methyltransferase in Chinese Han population of Shanghai area. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 17:421–423
Hon YY, Fessing MY, Pui CH, Relling MV, Krynetski EY, Evans WE (1999) Polymorphism of the thiopurine S-methyltransferase gene in African-Americans. Hum Mol Genet 8:371–376
Schwab M, Schaeffeler E, Marx C, Zanger U, Aulitzky W, Eichelbaum M (2001) Shortcoming in the diagnosis of TPMT deficiency in a patient with Crohn’s disease using phenotying only. Gastroenterology 121:498–499
Cheung ST, Allan RN (2003) Mistaken identity: misclassification of TPMT phenotype following blood transfusion. Eur J Gastroenterol Hepatol 15:1245–1247
Schaeffeler E, Stanulla M, Greil J et al (2003) A novel TPMT missense mutation associated with TPMT deficiency in a 5-year-old boy with ALL. Leukemia 17:1422–1424
Lindquist M, Haglund S, Almer S et al (2004) Identification of two novel sequence variants affecting thiopurine methyltransferase enzyme activity. Pharmacogenetics 14:261–265
Yan L, Zhang S, Eiff B, Szumlanski CL (2000) Thiopurine methyltransferase polymorphic tandem repeat: genotype-phenotype correlation analysis. Clin Pharmacol Ther 68:210–219
Alves S, Amorim A, Ferreira F, Prata MJ (2001) Influence of the variable number of tandem repeats located in the promoter region of the thiopurine methyltransferase gene on enzymatic activity. Clin Pharmacol Ther 70:165–174
Marinaki AM, Arenas M, Khan ZH et al (2003) Genetic determinants of the thiopurine methyltransferase intermediate activity phenotype in British Asians and Caucasians. Pharmacogenetics 13:97–105
Schütz E, Gummert J, Mohr F, Oellerich M (1993) Azathiopurine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet 13:341–346
Evans WE, Hon YY, Bomgaars L et al (2001) Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol 19:2293–2301
Black AJ, McLeod HL, Capeel HA et al (1998) Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from zazthiopurine. Ann Intern Med 129:716–718
Schwab M, Schäffeler E, Marx C et al (2002) Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics 12:429–436
Berkovitch M, Matsui D, Zipursky A et al (1996) Hepatotoxicity of 6-mercaptopurine in childhood acute lymphocytic leukemia: pharmacokinetic characteristics. Med Pediatr Oncol 26:85–89
Kader HA, Wenner WJ, Telega GW, Maller ES, Baldassano RN (2000) Normal thiopurine methyltransferase levels do not eliminate 6-mercaptopurine or azathioprine toxicity in children with inflammatory bowel disease. J Clin Gastroenterol 30:409–413
Dubinsky MC, Lamothe S, Yang HY et al (2000) Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 118:705–713
Alves S, Prata MJ, Ferreira F, Amorim A (1999) Thiopurine methyltransferase pharmacogenetics: alternative molecular diagnosis and preliminary data from Northern Portugal. Pharmacogenetics 9:257–261
Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM et al (2004) Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPA). Pharmacogenetics 14:181–187
Acknowledgements
This research was supported by the medical research foundation of PLA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xin, HW., Xiong, H., Wu, XC. et al. Relationships between thiopurine S-methyltransferase polymorphism and azathioprine-related adverse drug reactions in Chinese renal transplant recipients. Eur J Clin Pharmacol 65, 249–255 (2009). https://doi.org/10.1007/s00228-008-0589-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0589-0