Abstract
Purpose
Fulfilling bioequivalence criteria with highly variable drugs is difficult. The aim of this study was to compare the importance of sample size, intrasubject variability, and the point estimate of test and reference formulations with regard to meeting bioequivalence (BE) criteria [maximum observed plasma concentration (Cmax) and area under the concentration-time curve (AUC)].
Methods
We compared 137 pairs of data from BE studies with a conventional number of subjects, approximately 31–32 volunteers, developed in the last 10 years.
Results
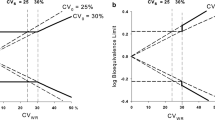
The third part of the studies failed to demonstrate BE, in part due to an unacceptable difference between the mean ratios (T/R) (18) but also due to high variability with small differences between formulations (17). Increasing the number of subjects is hard to justify, and expanding the confidence interval (CI) was insufficient for the most highly variable drugs.
Conclusions
Therefore, for low-variable drugs, the difference between formulations was the cornerstone of the fulfillment of BE criteria, but for highly variable drugs, the intrasubject coefficient of variability (ICV) was decisive. Our proposal is that for highly variable drugs that fall outside BE 90% CI limits could result in BE in the absence of formulation effect and maximal differences between formulations below 20%.









Similar content being viewed by others
References
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products–General Considerations, Office of Training and Communications, Division of Communications Management, Drug Information Branch, HFD-210, Rockville MD 20857, March 2003
U.S. Food and Drug Administration: Statistical approaches to establishing bioequivalence. Guidance for industry. Office of Training and Communications, Division of Communications Management, Drug Information Branch, HFD-210, Rockville MD 20857, January 2001
EMEA/CPMP/EWP/1401/98. Note for guidance on the investigation of bioavailability and bioequivalence. EMEA, London, 26 July 2001. http://www.emea.europa.eu/pdfs/human/qwp/140198enfin.pdf
EMEA/CHMP/EWP/200943/2007. Recommendation on the need for revision of (CHMP) “Note for guidance on the investigation of bioavailability and bioequivalence” CPMP/EWP/QWP/1401/98. http://www.emea.europa.eu/pdfs/human/ewp/20094307en.pdf
U.S. Food and Drug Administration: Bioequivalence Requirements for Highly Variable Drugs and Drug Products. Background Information for Advisory Committee Meeting, On April 11, 2004
CPMP/EWP/280/96. Note for Guidance on Modified Release Oral Dosage Forms and Transdermal Dosage Forms EMEA, London, 28 July 1999. http://www.tga.gov.au/docs/pdf/euguide/ewp/028096
World Medical Association Declaration OF Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Adopted by the 18th WMA General Assembly Helsinki, Finland, June 1964 and amended by the 29th WMA General Assembly, Tokyo, Japan, October 1975. 35th WMA General Assembly, Venice, Italy, October 1983
Julious SA (2004) Tutorial in biostatistics. Statistic Med 23:1921–1986
Boddy AW, Snikeris FC, Kringle RO, Wei GCG, Oppermann JA, Midha KK (1995) An approach for widening the bioequivalence acceptance limits in the case of highly variable drugs. Pharm Res 12:1865–1868
Karalis V, Macheras P, Symillides M (2005) Geometric mean ratio-dependent scaled bioequivalence limits with levelling-off properties. Eur J Pharm Sci 26:54–61
David BM. Highly variable drugs-bioequivalence issues: FDA proposal under consideration. Meeting of FDA Committee for Pharmaceutical Science. October 6, 2006. http://www.fda.gov/ohrms/dockets/ac/06/slides/2006–4241s2_5.htm
Health Canada, Ministry of Health, Guidance for Industry: Conduct and Analysis of Bioavailability and Bioequivalence Studies–Part A: Oral Dosage Formulations Used for Systemic Effects. 1992
Japan National Institute of Health, Division of Drugs (1997) Guideline for Bioequivalence Studies of Generic Drug Products
EMEA/CHMP/EWP/40326/2006. Questions & Answers on the Bioavailability and Bioequivalence Guideline. CHMP. Efficacy Working Party. Therapeutic Subgroup On Pharmacokinetics (EWP-PK), London 27 July 2006
U.S. Food and Drug Administration: Bioequivalence Requirements for Highly Variable Drugs and Drug Products. Background Information for Advisory Committee Meeting, October 6, 2006
Haidar SH, Davit B, Chen ML, Conner D, Lee L, Li QH, Lionberger R, Makhlouf F, Patel D, Schuirmann DJ, Yu LX (2007) Bioequivalence approaches for highly variable drugs and drug products. Pharm Res, Epub 2007 Sep 22
Acknowledgements
All authors have been working in the Phase I Clinical Trial Unit of Clinical Pharmacology Department, La Paz University Hospital, and the Department of Pharmacology & Therapeutics, School of Medicine, Autonomous University of Madrid, Spain.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ramirez, E., Guerra, P., Laosa, O. et al. The importance of sample size, log-mean ratios, and intrasubject variability in the acceptance criteria of 108 bioequivalence studies. Eur J Clin Pharmacol 64, 783–793 (2008). https://doi.org/10.1007/s00228-008-0476-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0476-8