Abstract
Objective
Inhaled corticosteroids may cause oropharyngeal side effects if deposited in the oropharynx in active form. Ciclesonide, an inhaled corticosteroid with low glucocorticoid receptor affinity, is activated primarily in the lung by esterases to an active metabolite, desisobutyryl-ciclesonide (des-CIC), with high glucocorticoid receptor affinity. We studied oropharyngeal deposition of ciclesonide, des-CIC, and budesonide.
Methods
In an open-label, randomized, two-treatment (administered in sequence), five-period study, 18 healthy subjects received 800 μg (ex-valve) inhaled ciclesonide via a hydrofluoroalkane-pressurized, metered-dose inhaler followed by 800 μg budesonide (Pulmicort) by a chlorofluorocarbon-pressurized, metered-dose inhaler (four puffs of 200 μg each, ex-valve) or vice versa. Oropharyngeal cavity rinsing was performed immediately, or 15, 30, 45, or 60 min after inhalation (one rinsing per study period), and the solutions were analyzed using liquid chromatography with tandem mass spectrometric detection.
Results
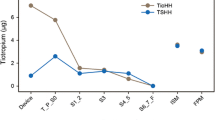
Ciclesonide and budesonide were detected in most oropharyngeal wash samples. Maximal concentration of each inhaled corticosteroid was reached immediately post-inhalation; maximal concentrations of ciclesonide and des-CIC were 30% and 0.67%, respectively, of budesonide. Oropharyngeal deposition of ciclesonide and budesonide decreased rapidly within 15 min post-inhalation, and less rapidly thereafter. Less than 10% of the residual ciclesonide in the oropharynx was converted to des-CIC. The molar dose-adjusted amount of des-CIC was 4% of budesonide (P < 0.0001). There were no significant adverse events.
Conclusion
Oropharyngeal deposition of des-CIC was more than one order of magnitude lower than that of budesonide when administered by the respective metered-dose inhalers. This may explain the low frequency of oropharyngeal side effects of ciclesonide in clinical studies.


Similar content being viewed by others
References
(2002) Ciclesonide: BY 9010, ciclesonide-DPI, ciclesonide-MDI, EL 876. Drugs R&D 3:407–410
Barry PW, O’Callaghan C (1996) Inhalational drug delivery from seven different spacer devices. Thorax 51:835–840
Bernstein JA, Noonan MJ, Rim C, Fish J, Kundu S, Williams J et al (2004) Ciclesonide has minimal oropharyngeal side effects in the treatment of patients with moderate-to-severe asthma. J Allergy Clin Immunol 113:S113
Bethke TD, Boudreau RJ, Hasselquist BE, Davidson P, Leach CL, Drollmann A et al (2002) High lung deposition of ciclesonide in 2D- and 3D-imaging. Eur Respir J 20(Suppl 38):109s
Boorsma M, Andersson N, Larsson P, Ullman A (1996) Assessment of the relative systemic potency of inhaled fluticasone and budesonide. Eur Respir J 9:1427–1432
Chapman KR, Patel P, Boulet LP, D’Urzo AD, Alexander M, Mehra S et al (2002) Efficacy and long-term safety of ciclesonide in asthmatic patients as demonstrated in a 52 week long study. Eur Respir J 20(Suppl 38):373s
Cumming RG, Mitchell P, Leeder SR (1997) Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med 337: 8–14
Engelstätter R, Banerji D, Steinijans VW, Wurst W (2004) Low incidence of oropharyngeal adverse events in asthma patients treated with ciclesonide: results from a pooled analysis. Am J Respir Crit Care Med 169:A92
Grahnen A, Jansson B, Brundin RM, Ling-Andersson A, Lonnebo A, Johansson M et al (1997) A dose–response study comparing suppression of plasma cortisol induced by fluticasone propionate from Diskhaler and budesonide from Turbuhaler. Eur J Clin Pharmacol 52:261–267
Hansel T, Engelstätter R, Benezet O, Kafé H, Ponitz HH, Cheung D et al (2003) Once daily ciclesonide (80 μg or 320 μg) is equally effective as budesonide 200 μg given twice daily: a 12-week study in asthma patients. Eur Respir J 22(Suppl 45):410s
Jackson LD, Polygenis D, McIvor RA, Worthington I (1999) Comparative efficacy and safety of inhaled corticosteroids in asthma. Can J Clin Pharmacol 6:26–37
Kelly HW (1999) Comparative potency and clinical efficacy of inhaled corticosteroids. Respir Care Clin N Am 5:537–553
Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ (2002) Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross-over study in healthy volunteers. Chest 122:510–516
Leech JA, Hodder RV, Ooi DS, Gay J (1993) Effects of short-term inhaled budesonide and beclomethasone dipropionate on serum osteocalcin in premenopausal women. Am Rev Respir Dis 148:113–115
Nave R, Bethke TD, van Marle SP, Zech K (2004) Pharmacokinetics of [14 C]ciclesonide after oral and intravenous administration to healthy subjects. Clin Pharmacokinet 43:479–486
Nave R, Fisher R, Zech K (2003) In vitro metabolism of ciclesonide in the human lung and liver as determined by use of precision-cut tissue slices. Am J Respir Crit Care Med 167(Suppl):A771
Newman S, Salmon A, Nave R, Drollmann A (2004) High lung deposition of 99mTc-labelled ciclesonide administered via HFA-MDI to asthma patients. Eur Respir J 24(suppl 48):583s
Newman SP, Brown J, Steed KP, Reader SJ, Kladders H (1998) Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devices. Chest 113:957–63
Pedersen S, Steffensen G, Ohlsson SV (1993) The influence of orally deposited budesonide on the systemic availability of budesonide after inhalation from a Turbuhaler. Br J Clin Pharmacol 36:211–214
Postma DS, Sevette C, Martinat Y, Schlösser N, Aumann J, Kafé H (2001) Treatment of asthma by the inhaled corticosteroid ciclesonide given either in the morning or evening. Eur Respir J 17:1083–1088
Rohatagi S, Appajosyula S, Derendorf H, Szefler S, Nave R, Zech K et al (2004) Risk–benefit value of inhaled glucocorticoids: a pharmacokinetic/pharmacodynamic perspective. J Clin Pharmacol 44:37–47
Rohatagi S, Arya V, Zech K, Nave R, Hochhaus G, Jensen BK et al (2003) Population pharmacokinetics and pharmacodynamics of ciclesonide. J Clin Pharmacol 43:365–378
Rohatagi S, Derendorf H, Zech K, Nave R, Banerji D (2003) PK/PD of inhaled corticosteroids: the risk/benefit of inhaled ciclesonide. J Allergy Clin Immunol 111:S218
Stoeck M, Riedel R, Hochhaus G, Haefner D, Masso JM, Schmidt B et al (2004) In vitro and in vivo anti-inflammatory activity of the new glucocorticoid ciclesonide. J Pharmacol Exp Ther 309:249–258
Szefler SJ, Herron J, Lloyd M, Rohatagi S, Williams JE, Kundu S et al (2003) High doses of the novel inhaled steroid ciclesonide have no effect on HPA-axis function in patients with moderate-to-severe persistent asthma. J Allergy Clin Immunol 111:S216
Thorsson L, Edsbäcker S, Conradson T-B (1994) Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J 7:1839–1844
Toogood JH, Jennings B, Baskerville J, Anderson J, Johansson SA (1984) Dosing regimen of budesonide and occurrence of oropharyngeal complications. Eur J Respir Dis 65:35–44
Toogood JH, White FA, Baskerville JC, Fraher LJ, Jennings B (1997) Comparison of the antiasthmatic, oropharyngeal, and systemic glucocorticoid effects of budesonide administered through a pressurized aerosol plus spacer or the Turbuhaler dry powder inhaler. J Allergy Clin Immunol 99:186–193
Weinbrenner A, Hüneke D, Zschiesche M, Engel G, Timmer W, Steinijans VW et al (2002) Circadian rhythm of serum cortisol after repeated inhalation of the new topical steroid ciclesonide. J Clin Endocrinol Metab 87:2160–2163
Williamson IJ, Matusiewicz SP, Brown PH, Greening AP, Crompton GK (1995) Frequency of voice problems and cough in patients using pressurized aerosol inhaled steroid preparations. Eur Respir J 8:590–592
Acknowledgements
The following investigators participated in this study: Manfred Hartmann, MD, MSc, Bernhard Hauns, MD, Ulrich Kilian, MD, and Wolfgang Timmer, MD. The authors would like to thank Mr. Werner Meyer (MDS Pharmaservices, Fehraltorf, Switzerland) for performing the bioanalytical work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nave, R., Zech, K. & Bethke, T.D. Lower oropharyngeal deposition of inhaled ciclesonide via hydrofluoroalkane metered-dose inhaler compared with budesonide via chlorofluorocarbon metered-dose inhaler in healthy subjects. Eur J Clin Pharmacol 61, 203–208 (2005). https://doi.org/10.1007/s00228-005-0910-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0910-0