Abstract
Objectives
Adverse drug interactions increase morbidity and mortality. To prevent these, situations leading to adverse prescriptions must be clarified. This study quantifies and analyses prescriptions with potential adverse drug interactions in primary health care in the north of France over a 3-month period.
Methods
All prescriptions administered between 1 January and 31 March 1999 were analysed to identify potential interactions amongst drugs appearing on the same prescription sheet. The regional French healthcare database was compiled to further classify contra-indications.
Results
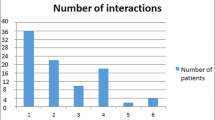
There were 5,358,374 prescriptions administered to 44% of the overall population of the Nord-Pas de Calais area (1,754,372 patients/3,990,167 general population). There were 14,390 prescriptions classified as either absolute (26%) or relative contraindications (74%). Nine drug categories accounted for most of the absolute contraindications: dopaminergic antiparkinsonians, neuroleptic agents, migraine treatments (such as ergot alkaloids, sumatriptan and other triptan derivatives), prokinetic drugs (cisapride), antibacterial drugs (macrolides), antifungals (imidazoles), antiarrhythmics, betablockers and analgesics (opioids and floctafenine). In 54% of patients exposed, the incurred risk was either QT prolongation/Torsade de Pointes or antagonism of dopaminergic antiparkinson agents with dopamine receptor antagonists prescribed as antipsychotic agents.
Conclusions
Among a non-selected population of ambulatory outpatients, the number of quarterly prescriptions with contra-indications with potentially harmful drug interaction was 27 in 10,000 prescriptions. This would extrapolate to nearly 200,000 contra-indications on the same-prescription sheets in France in the first quarter of 1999.

Similar content being viewed by others
References
Lazarou J, Pomeranz BH, Corey PN (1998) Incidence of adverse drug reactions in hospitalized patients. A meta-analysis of prospectives studies. JAMA 279:1200–1205
Fattinger K, Roos M, Vergères P, et al. (2000) Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. J Clin Pharmacol 49:158–167
Borda JP, Sloan D, Jick H (1968) Assessment of adverse reactions within a drug surveillance program. JAMA 205:645–647
Boston Collaborative Drug Surveillance Program (1972) Adverse drug reactions. JAMA 220:1238–1239
Mok H, Mulpeter K, O’connor P, Feely J (1991) Drug–drug interactions in the hospital. Irish Med J 84:26
Stanton LA, Peterson GM, Rumble RH (1994) Drug-related admissions to an Australian hospital. J Clin Pharmacol Ther 19:341–347
Steven ID, Malpass A, Moller J, Runciman WB, Helps SC (1999) Towards safer drug use in general practice. J Qual Clin Practice 19:47–50
Beers MH, Storrie M, Lee G (1990) Potential adverse drug interactions in the emergency room. Ann Intern Med 112:61–64
Jankel CA, Fitterman LK (1993) Epidemiology of drug-drug interactions as a cause of hospital admissions. Drug Saf 9:51–59
Classen K, Pestonik S, Evans S, Lloyd J, Burke J (1997) Adverse drug events in hospitalised patients excess length of stay, extra costs, and attributable mortality. JAMA 227:301–306
Adson DE, Crow SJ, Meller WH, Magraw RM (1998) Potential drug–drug interactions on a tertiary-care hospital consultation-liaison psychiatry service. Psychosomatics 39:360–365
Cadieux RJ (1989) Drug interactions in the elderly: how multiple drug use increases risk exponentially. Postgrad Med 86:179–186
Costa AJ (1991) Potential drug interactions in an ambulatory geriatric population. Fam Pract 8:234–236
Goldberg RM, Mabee J, Chan L, Wong S (1996) Drug–drug and drug–disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med 14:447–450
Hamilton RA, Gordon T (1992) Incidence and cost of hospital admissions secondary to drug interactions involving theophylline. Ann Pharmacother 26:1507–1511
Hansten PD (1991) Drug interactions with antisecretory drugs. Aliment Pharmacol Ther 5[Suppl 1]:121–128
Jankel CA, McMillan JA, Martin BC (1994) Effect of drug interactions on outcomes of patients receiving warfarin or theophylline. Am J Hosp Pharm 51:661–666
Johnson Z, Dack P (1992) Prescribing patterns for combinations of warfarin and potentially dangerous interacting drugs in a large general practice population. Pharmacoepidemiol Drug Saf 1:119–123
Gouveia WA, Miller RR (1978) Drug interaction screening. A screen or a sieve? Am J Hosp Pharm 35:667
Jinks MJ, Hansten PD, Hirschman JL (1979) Drug interaction exposure in an ambulatory medicaid population. Am J Hosp Pharm 36:923–927
Linnarson R (1993) Drug interactions in primary health care. A retrospective database study and its implications for the design of a computerized decision support system. Scand J Prim Health Care 11:181–186
Mitchell GW, Stanaszek WF, Nichois NB (1979) Documenting drug–drug interactions in ambulatory patients. Am J Hosp Pharm 36:653–657
Paille F, Pissochet P (1995) Drug interactions in primary health care: prospective study of 896 patients treated for hypertension. Therapie 50:253–258
Paulet N, Bury PC, Needleman M, Raymond K (1982) Drug interactions. A study and evaluation of their incidence in Victoria. Med J Aust 1:80–81
Remy N, Lapeyre-Mestre M, Bareille MP, Bagheri H, Montastruc JL (2000) Drug interactions: a prospective pilot study in primary health care. Therapie 55:395–398
Shinn AF, Shrewsbury RB, Anderson KW (1983) Development of a computerized drug interaction database (Medicom) for use in a patient specific environment. Drug Inf J 17:205–210
Anonymous (1999) Dictionnaire Vidal. Editions du Vidal, Paris (http://www.vidalpro.net/)
Seroussi B, Gerondeau N, Morice V, Boisvieux JF (1997) Comparative evaluation of French drug data banks. Therapie 52:207–212
Jankel CA, Speedie SM (1990) Detecting drug interactions: a review of the literature. Ann Pharmacother 24:982–989
Vargas E, Navarro MI, Laredo L, Garcia-Arenillas M, Garcia-Mateos M, Moreno A (1997) Effect of drug interactions on the development of adverse drug reactions. Clin Drug Invest 13:282–289
Comet D, Casajuana J, Bordas JM, Fuentes MAAJA, Nunez B, Pou R (1997) Drug interactions in chronic prescription. Study in four primary care centers. Atencion Primaria 20:31–34
Davidson KW, Kahn A, Price RD (1987) Reduction of adverse drug reactions by computerized drug interaction screening. J Fam Pract 25:371–375
Author information
Authors and Affiliations
Corresponding author
Additional information
The work is also attributed to the following institutions: Union Regionale des Caisses d’Assurance Maladie du Nord Pas de Calais. Villeneuve d’Ascq, France; and Department of Pharmacology University of Lille, France.
Rights and permissions
About this article
Cite this article
Guédon-Moreau, L., Ducrocq, D., Duc, MF. et al. Absolute contraindications in relation to potential drug interactions in outpatient prescriptions: analysis of the first five million prescriptions in 1999. Eur J Clin Pharmacol 59, 689–695 (2003). https://doi.org/10.1007/s00228-003-0684-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0684-1