Abstract
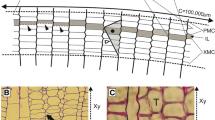
Spiral grain in trees is formed during the process of cell division and maturation within the vascular cambium (sensu lato). Much effort in the past half century has been put into elucidating the mechanism(s) involved. The most accepted view is that the dominant factor involves the pseudotransverse cell divisions during anticlinal (multiplicative) division and subsequent intrusive growth of the daughter cells, the slant of the partition (S or Z) and the direction of the intrusion being statistically biased to the left or to the right. A strong correlation is known to exist between slant direction and its frequency of occurrence on the one hand and the rate of change of spiral grain angle on the other hand. It is thus enticing to see the dominance of the orientation of the slant of the partition during pseudotransverse anticlinal cell division as the causative factor in the formation of spiral grain; the visual evidence seems clear. However, it will be shown that there is a constant, incessant tendency of all maturing cells (which are predominantly the result of periclinal divisions) to change their orientation in a given direction; this tendency will be modulated as to magnitude and direction by certain prevailing physical parameters of the system during the period of maturation. Thus it is concluded that neither the slant of pseudotransverse divisions nor other “isolated events” (imperfect periclinal division, biased intrusive growth) are causative, but that they rather result from the fact that there is a radial gradient of the inclination angle (in the tangential plane) of fusiform cells, i.e. from the general tendency of a maturing cell to take on a preferred inclination with respect to the cell which immediately preceded it in its file. Growth stress patterns in trees have also been extensively investigated in the past half century. It is shown that the development of these stresses and the formation of spiral grain are just two aspects of the same process occurring throughout the cambial zone during cell maturation. Models are presented to justify this claim which accord with reported patterns of growth stress and spiral grain in conifers.






Similar content being viewed by others
References
Archer RR (1979) On the distribution of tree growth stresses. Part III: the case of inclined grain. Wood Sci Technol 13:67–78
Archer RR (1987a) On the origin of growth stresses in trees. Part 1: micro mechanics of the developing cell wall. Wood Sci Technol 21:139–154
Archer RR (1987b) Growth stresses and strains in trees. Springer, Berlin Heidelberg New York
Archer RR (1989) On the origin of growth stresses in trees. Part 2: stresses generated in a tissue of developing cells. Wood Sci Technol 23:311–322
Bamber RK (1979) The origin of growth stresses. Forpride Digest 8:75–79
Bannan MW (1964) Tracheid size and anticlinal divisions in the cambium of Pseudotsuga. Can J Bot 42:603–631
Bannan MW (1966) Spiral grain and anticlinal divisions in the cambium of conifers. Can J Bot 44:1515–1538
Barber NF, Meylan BA (1964) The anisotropic shrinkage of wood: a theoretical model. Holzforschung 18:146–156
Barnett JR, Bonham VA (2004) Cellulose microfibril angle in the cell wall of wood fibres. Biol Rev 79:461–472
Barrett JD, Schniewind AP (1973) Three-dimensional finite element models of cylindrical wood fibers. Wood Fiber 5:215–225
Bodig J, Jayne BA (1982) Mechanics of wood and wood composites. Van Nostrand Reinhold, New York
Bonham VA, Barnett JR (2001) Fibre length and microfibril angle in silver birch (Betula pendula Roth). Holzforschung 55:159–162
Boyd JD (1972) Tree growth stresses. V. Evidence of an origin in differentiation and lignification. Wood Sci Technol 6:251–262
Braam J (2005) In touch: plant responses to mechanical stimuli. New Phytol 165:373–389
Brown CL, Sax K (1962) The influence of pressure on the differentiation of secondary tissues. Am J Bot 49:683–691
Champion HG (1925) Contributions toward a knowledge of twisted fibre in trees. Indian For Rec 11(P.II):11–80
Cown DJ, Young GD, Kimberley MO (1991) Spiral grain patterns in plantation-grown Pinus radiata. NZ J For Sci 21:206–216
Danborg F (1994) Spiral grain in plantation trees of Picea abies. Can J For Res 24:1662–1671
Donaldson LA (1992) Within and between tree variation in microfibril angle in Pinus radiata. NZ J For Sci 22:77–86
Donaldson L, Xu P (2005) Microfibril orientation across the secondary cell wall of Radiata pine tracheids. Trees 19:644–653
Eklund L, Säll H, Linde S (2003) Enhanced growth and ethylene increases spiral grain formation in Picea abies and Abies balsamea trees. Trees 17:81–86
Forest Products Laboratory (1999) Wood handbook – wood as an engineering material, Gen. Tech. Rep. FPL-GTR-113, U.S. Department of Agriculture
Fournier M, Bardonne PA, Guitard D, Okuyama T (1990) Growth stress patterns in tree stems. Wood Sci Technol 24:131–142
Gartner BL (1997) Trees have higher longitudinal growth strains in their stems than in their roots. Int J Plant Sci 158:418–423
Gjerdrum P, Säll H, Storø HM (2002) Spiral grain in Norway spruce: constant change rate in grain angle in Scandinavian sawlogs. Forestry 75:163–170
Gould SJ, Lewontin RC (1979) The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B 205:581–598
Gray DG, Kam A (1997) Chiral characteristics of thin wood sections. Holzforschung 51:1–5
Harris JM (1969) On the causes of spiral grain in corewood of radiata pine. NZ J Bot 7:189–213
Harris JM (1973) Spiral grain and xylem polarity in radiata pine: microscopy of cambial reorientation. NZ J For Sci 3:363–378
Harris JM (1981) Spiral grain formation. In: Barnett JR (ed) Xylem cell development. Castle House, Tunbridge Wells, pp 256–272
Harris JM (1989) Spiral grain and wave phenomena in wood formation. Springer, Berlin Heidelberg New York
Hartig R (1895) Über den Drehwuchs der Kiefer. Forst-Naturwiss Z 4:313–326
Hejnowicz Z (1961) Anticlinal division, intrusive growth, and loss of fusiform initials in nonstoried cambium. Acta Soc Bot Pol 30:729–748
Hejnowicz Z (1968) The structural mechanism involved in the changes of grain in timber. Acta Soc Bot Pol 37:347–365
Hejnowicz Z, Krawczyszyn J (1969) Oriented morphogenetic phenomena in cambium of broadleaved trees. Acta Soc Bot Pol 38:547–560
Hejnowicz Z, Romberger JA (1979), The common basis of wood grain figures in the systematically changing orientation of cambial fusiform cells. Wood Sci Technol 13:89–96
Hejnowicz Z, Zagorska-Marek B (1974) Mechanism of changes in grain inclination in wood produced by storeyed cambium. Acta Soc Bot Pol 43:381–398
Hori R, Suzuki H, Kamiyama T, Sugiyama J (2003) Variation of microfibril angles and chemical composition: implications for functional properties. J Mat Sci Lett 22:963–966
Houkal D (1982) Spiral grain in Pinus oocarpa. Wood Fiber 14:320–330
Jones BE (1963) Cell adjustments accompanying the development of spiral grain in a specimen of Pseudotsuga taxifolia. Br Common For Rev 42:152–158
Jones RM (1999) Mechanics of composite materials, 2nd edn. Taylor & Francis, Philadelphia
Kirschner H, Sachs T, Fahn A (1971) Secondary xylem reorientation as a special case of vascular tissue differentiation. Isr J Bot 20:184–198
Kohl EJ (1933) An explanation of the cause of spiral grain in trees. Science 78:58–59
Kollmann FFP, Côté Jr. WA (1968) Principles of wood science and technology. Springer, Berlin Heidelberg New York
Kozlowski TT, Pallardy SG (1996) Physiology of woody plants, 2nd edn. Academic, San Diego
Krempl H (1970) Untersuchungen über den Drehwuchs bei Fichte (Picea abies Karst.). Mitt Forst Bundes-Versuchsanst Wien 89, 118 pp
Kubler H (1959) Studien über Wachstumsspannungen des Holzes. Zweite Mitteilung: Die Spannungen in Faserrichtung. Holz Roh- Werkst 17:44–54
Kubler H (1987) Growth stresses in trees and related wood properties. For Abstr 48:131–189
Kubler H (1991) Function of spiral grain in trees. Trees 5:125–135
Larson PR (1994) The vascular cambium: development and structure. Springer, Berlin Heidelberg New York
Leelavanichkul S, Cherkaev A (2004) Why grain in tree trunks spirals: a mechanical perspective. Struct Multidisc Optim 28:127–135
Lintilhac PM, Vesecky TB (1984) Stress-induced alignment of division plane in plant tissues grown in vitro. Nature 307:363–364
Little CHA, Savidge RA (1987) The role of plant growth regulators in forest tree cambial growth. Plant Growth Regul 6:137–169
Lynch TM, Lintilhac PM (1997) Mechanical signals in plant development: a new method for single cell studies. Dev Biol 181:246–256
Mark RE (1967) Cell wall mechanics of tracheids. Yale University Press, New Haven
Mark RE, Gillis PP (1970) New models in cell wall mechanics. Wood Fiber 2:79–95
Mark RE, Gillis PP (1983) Mechanical properties of fibers. In: Mark RE (ed) Handbook of physical and mechanical testing of paper and paperboard. Marcel Dekker, pp 409–495
Megraw RE (1985) Wood quality factors in loblolly pine. Tappi Press, Atlanta
Meylan BA, Butterfield BG (1972) Three-dimensional structure of wood: a scanning electron microscope study. Syracuse University Press, Syracuse
Meylan BA, Butterfield BG (1978) Helical orientation of the microfibrils in tracheids, fibers and vessels. Wood Sci Technol 12:219–222
Northcott PL (1957) Is spiral grain the normal growth pattern? For Chron 33:333–352
Noskowiak AF (1963) Spiral grain in trees: a review. For Prod J 13:266–275
Okuyama T, Sasaki Y, Kikata Y (1981) The seasonal change in growth stress in the tree trunk. Mokuzai Gakkaishi 27:350–355
Okuyama T, Kawai A, Kikata Y (1983) Growth stresses and uneven gravitational-stimulus in trees containing reaction wood. Mokuzai Gakkaishi 29:190–196
Page DH, Seth RS (1980) The elastic modulus of paper. II. The importance of fiber modulus, bonding, and fiber length. Tappi 63(6):113–116
Page DH, El-Hosseiny F, Lancaster APS (1977) Elastic modulus of single wood pulp fibers. Tappi 60(4):114–117
Pope DJ, Marcroft JP, Whale LRJ (2005) The effect of global slope of grain on the bending strength of scaffold boards. Holz Roh- Werkst 63:321–326
Preston RD (1949) Spiral structure and spiral growth: the development of spiral grain in conifers. Forestry 23:48–55
Savidge RA, Farrar JL (1984) Cellular adjustments in the vascular cambium leading to spiral grain formation in conifers. Can J Bot 62:2872–2879
Schniewind AP, Barrett JD (1969) Cell-wall model with complete shear restraint. Wood Fiber 1:205–214
Schulgasser K, Page DH (1988) The influence of transverse fiber properties on the in-plane elastic behavior of paper. Comp Sci Technol 32:279–292
Schulgasser K, Witztum A (2004) The hierarchy of chirality. J Theoret Biol 230:281–288
Skatter S, Archer RR (2001) Residual stresses caused by growth stresses within a stem with radially varying spiral grain angle - two numerical solution approaches: (1) finite element method and (2) transfer matrix method. Wood Sci Technol 35:57–71
Skatter S, Kučera B (1997) Spiral grain – an adaptation of trees to withstand stem breakage caused by wind-induced torsion. Holz Roh- Werkst 55:207–213
Skatter S, Kučera B (1998) The cause of the prevalent directions of the spiral grain patterns in conifers. Trees 12:265–273
Skatter S, Kucera B (2000) Tree breakage from torsional wind loading due to crown asymmetry. For Ecol Manage 135:97–103
Smith LG (2001) Plant cell division: building walls in the right places. Nat Rev Mol Cell Biol 2:33–39
Telewski FW (1995) Wind-induced physiological and developmental responses in trees. In: Coutts MP, Grace J (eds) Wind and trees. Cambridge University Press, Cambridge, pp 237–263
Telewski FW, Jaffe MJ (1986) Thigmomorphogenesis: field and laboratory studies of Abies fraseri in response to wind or mechanical perturbation. Physiol Plant 66:211–218
Thair BW, Steeves TA (1976) Response of the vascular cambium to reorientation in patch grafts. Can J Bot 54:361–373
Theophrastus (1916) Enquiry into plants (trans. A. Hort), Book V. Harvard University Press, Cambridge, p 427
Thibaut B, Gril J, Fournier M (2001) Mechanics of wood and trees: some new highlights for an old story. C R Acad Sci Paris t 329(Série II b):701–716
Thunell B (1951) Über die Drehwüchsigkeit. Holz Roh- Werkst 8:293–297
Vité JP (1958) Über die transpirationsphysiologische Bedeutung des Drehwuchses bei Nadelhölzern. Forstwiss Cbl 77:193–203
Vité JP, Rudinsky JA (1959) The water-conducting systems in conifers and their importance to the distribution of trunk injected chemicals. Contr Boyce Thompson Inst 20:27–38
Wentworth CK (1931) Twist in the grain of coniferous trees. Science 73:192
Włoch W (1981) Nonparallelism of cambium cells in neighboring rows. Acta Soc Bot Pol 50:625–636
Włoch W, Mazur E, Beltowski M (2002) Formation of spiral grain in the wood of Pinus sylvestris L. Trees 16:306–312
Xia Z, Okabe T, Curtin WA (2002) Shear-lag versus finite element models for stress transfer in fiber-reinforced materials. Comp Sci Technol 62:1141–1149
Yamamoto H (1998), Generation mechanism of growth stresses in wood cell walls: roles of lignin deposition and cellulose microfibril during cell wall maturation. Wood Sci Technol 32:171–182
Yamamoto H, Okuyama T, Yoshida M (1995) Generation process of growth stresses in cell walls VI. Mokuzai Gakkaishi 41:1–8
Yamamoto H, Kojima Y, Okuyama T, Abasolo WP, Gril J (2002), Origin of the biomechanical properties of wood related to the structure of the multi-layered cell wall. J Biomech Eng 124:432–440
Zagorska-Marek B, Little CHA (1986) Control of fusiform initial orientation in the vascular cambium of Abies balsamea stems by indol-3-ylacetic acid. Can J Bot 64:1120–1127
Zobel BJ, van Buijtenen JP (1989) Wood variation: its causes and control. Springer, Berlin Heidelberg New York
Acknowledgements
This research was partially supported by the Israel Science Foundation (Grant no. 301/99).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulgasser, K., Witztum, A. The mechanism of spiral grain formation in trees. Wood Sci Technol 41, 133–156 (2007). https://doi.org/10.1007/s00226-006-0100-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-006-0100-y