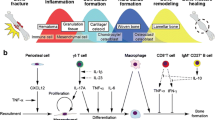
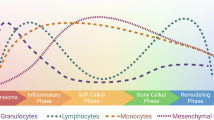
Abstract
The immune system is an active component of bone repair. Mast cells influence the recruitment of macrophages, osteoclasts and blood vessels into the repair tissue. We hypothesized that if mast cells and other immune cells are sensitized to recognize broken bone, they will mount an increased response to subsequent fractures that may be translated into enhanced healing. To test this, we created a bone defect on the left leg of anesthetized mice and 2 weeks later, a second one on the right leg. Bone repair in the right legs was then compared to control mice that underwent the creation of bilateral window bone defects at the same time. Mice were euthanized at 14 and 56 days. Mineralized tissue quantity and morphometric parameters were assessed using micro-CT and histology. The activity of osteoblasts, osteoclasts, vascular endothelial cells, mast cells, and macrophages was evaluated using histochemistry. Our main findings were (1) no significant differences in the amount of bone produced at 14- or 56 days post-operative between groups; (2) mice exposed to subsequent fractures showed significantly better bone morphometric parameters after 56 days post-operative; and (3) significant increases in the content of blood vessels, osteoclasts, and the number of macrophages in the subsequent fracture group. Our results provide strong evidence that a transient increase in the inflammatory state of a healing injury promotes faster bone remodelling and increased neo-angiogenesis. This phenomenon is also characterized by changes in mast cell and macrophage content that translate into more active recruitment of mesenchymal stromal cells.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are openly available in McGill University’s Dataverse Institutional Repository (https://dataverse.scholarsportal.info/dataverse/mcgill).
Code Availability
Not applicable.
References
Schneider PS, Sandman E, Martineau PA (2018) Osteoimmunology: effects of standard orthopaedic interventions on inflammatory response and early fracture healing. J Am Acad Orthop Surg 26(10):343–352
Harper D, Young A, McNaught C-E (2014) The physiology of wound healing. Surgery (Oxf) 32(9):445–450
Ramirez-Garcia-Luna JL, Wong TH, Chan D, Al-Saran Y, Awlia A, Abou-Rjeili M et al (2019) Defective bone repair in diclofenac treated C57Bl6 mice with and without lipopolysaccharide induced systemic inflammation. J Cell Physiol 234(3):3078–3087
Bodnar RJ (2015) Chemokine regulation of angiogenesis during wound healing. Adv Wound Care (New Rochelle) 4(11):641–650
Claes L, Recknagel S, Ignatius A (2012) Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 8(3):133–143
Li J, Tan J, Martino MM, Lui KO (2018) Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol 9:585
Mansour A, Abu-Nada L, Al-Waeli H, Mezour MA, Abdallah M-N, Kinsella JM et al (2019) Bone extracts immunomodulate and enhance the regenerative performance of dicalcium phosphates bioceramics. Acta Biomater 89:343–358
Medhat D, Rodríguez CI, Infante A (2019) Immunomodulatory effects of MSCs in bone healing. Int J Mol Sci 20(21):5467
Wu T, Zhang J, Wang B, Sun Y, Liu Y, Li G (2018) Staphylococcal enterotoxin C2 promotes osteogenesis of mesenchymal stem cells and accelerates fracture healing. Bone Jt Res 7(2):179–186
Andersson C, Tufvesson E, Diamant Z, Bjermer L (2016) Revisiting the role of the mast cell in asthma. Curr Opin Pulm Med 22(1):10–17
Behrends DA, Cheng L, Sullivan MB, Wang MH, Roby GB, Zayed N et al (2014) Defective bone repair in mast cell deficient mice with c-Kit loss of function. Eur Cell Mater 28:209–221; discussion 221–222
Kroner J, Kovtun A, Kemmler J, Messmann JJ, Strauss G, Seitz S et al (2017) Mast cells are critical regulators of bone fracture-induced inflammation and osteoclast formation and activity. J Bone Miner Res 32(12):2431–2444
Ramirez-GarciaLuna JL, Chan D, Samberg R, Abou-Rjeili M, Wong TH, Li A et al (2017) Defective bone repair in mast cell-deficient Cpa3Cre/+ mice. PLoS ONE 12(3):e0174396
Behrends DA, Hui D, Gao C, Awlia A, Al-Saran Y, Li A et al (2017) Defective bone repair in C57Bl6 mice with acute systemic inflammation. Clin Orthop Relat Res 475(3):906–916
Park H, Collignon A-M, Lepry WC, Ramirez-GarciaLuna JL, Rosenzweig DH, Chaussain C et al (2020) Acellular dense collagen-S53P4 bioactive glass hybrid gel scaffolds form more bone than stem cell delivered constructs. Mater Sci Eng C 120:111743
Castano D, Comeau-Gauthier M, Ramirez-GarciaLuna JL, Drager J, Harvey E, Merle G (2019) Noninvasive localized cold therapy: a new mode of bone repair enhancement. Tissue Eng A 25(7–8):554–562
Comeau-Gauthier M, Tarchala M, Luna JLR-G, Harvey E, Merle G (2020) Unleashing β-catenin with a new anti-Alzheimer drug for bone tissue regeneration. Injury 51(11):2449–2459
Drager J, Ramirez-GarciaLuna JL, Kumar A, Gbureck U, Harvey EJ, Barralet JE (2017) Hypoxia biomimicry to enhance monetite bone defect repair. Tissue Eng A 23(23–24):1372–1381
Hemmatian H, Laurent MR, Ghazanfari S, Vanderschueren D, Bakker AD, Klein-Nulend J et al (2017) Accuracy and reproducibility of mouse cortical bone microporosity as quantified by desktop microcomputed tomography. PLoS ONE 12(8):e0182996
Cooke ME, Ramirez-GarciaLuna JL, Rangel-Berridi K, Park H, Nazhat SN, Weber MH et al (2020) 3D printed polyurethane scaffolds for the repair of bone defects. Front Bioeng Biotechnol 8:1. https://doi.org/10.3389/fbioe.2020.557215/abstract (cited 23 Sep 2020)
Henderson JE, Gao C, Harvey EJ (2011) Skeletal Phenotyping in rodents: tissue isolation and manipulation. In: Osteoporosis research. Springer, London, pp 13–28. https://doi.org/10.1007/978-0-85729-293-3_2 (cited 7 Feb 2018)
Griffanti G, Rezabeigi E, Li J, Murshed M, Nazhat SN (2020) Rapid biofabrication of printable dense collagen bioinks of tunable properties. Adv Funct Mater 30(4):1903874
Gao C, Harvey EJ, Chua M, Chen BP, Jiang F, Liu Y et al (2013) MSC-seeded dense collagen scaffolds with a bolus dose of VEGF promote healing of large bone defects. Eur Cell Mater 26:195–207; discussion 207
Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH et al (2005) Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol 25(14):6199–6210
Moraes C, Simon AB, Putnam AJ, Takayama S (2013) Aqueous two-phase printing of cell-containing contractile collagen microgels. Biomaterials 34(37):9623–9631
Ponzetti M, Rucci N (2019) Updates on osteoimmunology: what’s new on the cross-talk between bone and immune system. Front Endocrinol (Lausanne) 10:236
Tsukasaki M, Takayanagi H (2019) Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol 19(10):626–642
Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J et al (2003) Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 18(9):1584–1592
Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y et al (2016) IL-17-producing γδ T cells enhance bone regeneration. Nat Commun 7:10928
Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann J-E, Frisch J-T et al (2011) Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res 26(1):113–124
Frost HM (1983) The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J 31(1):3–9
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ et al (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17(10):1231–1234
Batoon L, Millard SM, Raggatt LJ, Pettit AR (2017) Osteomacs and bone regeneration. Curr Osteoporos Rep 15(4):385–395
Miron RJ, Bosshardt DD (2016) OsteoMacs: key players around bone biomaterials. Biomaterials 82:1–19
Tsukasaki M, Hamada K, Okamoto K, Nagashima K, Terashima A, Komatsu N et al (2017) LOX fails to substitute for RANKL in osteoclastogenesis. J Bone Miner Res 32(3):434–439
Kitaura H, Sands MS, Aya K, Zhou P, Hirayama T, Uthgenannt B et al (2004) Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo. J Immunol 173(8):4838–4846
Nakashima T, Hayashi M, Takayanagi H (2012) New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab 23(11):582–590
Alshahrani NS, Abu-Nada L, Ramirez Garcia-Luna JL, Al-Hamed FS, Alamri A, Makhoul NM et al (2020) Ranitidine impairs bone healing and implant osseointegration in rats’ tibiae. J Oral Maxillofac Surg 78(11):1943–1952
Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA (2001) Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-α. J Immunol 167(7):4008–4016
Wernersson S, Pejler G (2014) Mast cell secretory granules: armed for battle. Nat Rev Immunol 14(7):478–494
Dahlin JS, Hallgren J (2015) Mast cell progenitors: origin, development and migration to tissues. Mol Immunol 63(1):9–17
Zhou Y, Pan P, Yao L, Su M, He P, Niu N et al (2010) CD117-positive cells of the heart: progenitor cells or mast cells? J Histochem Cytochem 58(4):309–316
Štefková K, Procházková J, Pacherník J (2015) Alkaline phosphatase in stem cells. Stem Cells Int 2015:e628368
Stegen S, van Gastel N, Carmeliet G (2015) Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone 70:19–27
Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A (2014) Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 32(6):1380–1389
Viswanathan C, Kulkarni R, Bopardikar A, Ramdasi S (2017) Significance of CD34 negative hematopoietic stem cells and CD34 positive mesenchymal stem cells—a valuable dimension to the current understanding. Curr Stem Cell Res Ther 12(6):476–483
Drager J, Harvey EJ, Barralet J (2015) Hypoxia signalling manipulation for bone regeneration. Expert Rev Mol Med 17:e6
Hardy E, Fernandez-Patron C (2020) Destroy to rebuild: the connection between bone tissue remodeling and matrix metalloproteinases. Front Physiol 11:47
James EA, Pietropaolo M, Mamula MJ (2018) Immune recognition of β-cells: neoepitopes as key players in the loss of tolerance. Diabetes 67(6):1035–1042
Verdegaal EME, van der Burg SH (2017) The potential and challenges of exploiting the vast but dynamic neoepitope landscape for immunotherapy. Front Immunol 8:1113
Phung TN, Lenkiewicz E, Malasi S, Sharma A, Anderson KS, Wilson MA et al (2020) Unique genomic and neoepitope landscapes across tumors: a study across time, tissues, and space within a single lynch syndrome patient. Sci Rep 10(1):12190
Elango J, Robinson J, Zhang J, Bao B, Ma N, de Val JEMS et al (2019) Collagen peptide upregulates osteoblastogenesis from bone marrow mesenchymal stem cells through MAPK-Runx2. Cells 8(5):446
Funck-Brentano T, Lin H, Hay E, Kioon M-DA, Schiltz C, Hannouche D et al (2012) Targeting bone alleviates osteoarthritis in osteopenic mice and modulates cartilage catabolism. PLoS ONE 7(3):e33543
Acknowledgements
The work was performed in the Bone Engineering and the Regenerative Orthopaedics and Innovation Laboratories of the Research Institute-McGill University Health Centre, which are a Fonds de Recherche Québec Santé (FRQS) sponsored Centre de Recherche. The Bone Engineering Labs are supported in part by the Fonds de Recherche Québec Santé-Réseau de recherche en santé buccodentaire et osseuse (FRQS-RSBO). JRGL and KRB are scholars of the Mexican National Council for Science and Technology (CONACYT). JRGL also receives a doctoral fellowship from FRSQ. PAM is FRQS Chercheur boursier clincien Junior 2. The authors would like to acknowledge the contributions of Dr. Christopher Moraes and Miss. Mira Abou-Rjeili for the in vitro studies.
Funding
Canadian Institutes of Health Research (CIHR) and Fonds de recherche du Québec – Santé (FRQS).
Author information
Authors and Affiliations
Contributions
Conceptualization: JR-GL, JEH, PAM. Data acquisition and curation: JR-G, KR-B, O-OU. Formal analysis: JR-G, DHR, RG, PAM. Funding acquisition: JEH, PAM. Methodology: DHR, JEH, PAM. Resources: DHR, JEH, RG, PAM. Supervision: DHR, JEH, PAM. Manuscript writing, review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical Approval
McGill MGH Facility Animal Care Committee (7016).
Human and Animal Rights
All live animal procedures were conducted in accordance with a protocol approved by McGill MGH Facility Animal Care Committee (7016) and in keeping with the guidelines of the Canada Council on Animal Care.
Informed Consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramirez-GarciaLuna, J.L., Rangel-Berridi, K., Olasubulumi, OO. et al. Enhanced Bone Remodeling After Fracture Priming. Calcif Tissue Int 110, 349–366 (2022). https://doi.org/10.1007/s00223-021-00921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00921-5