Abstract
Dual-energy X-ray absorptiometry (DXA) is the gold standard for diagnosing osteoporosis; it is generally recommended in men ≥ 70 and women ≥ 65 years old. Therefore, assessment of clinical risk factors for osteoporosis is very important in individuals under the recommended age for DXA. Here, we examine the diagnostic performance of machine learning-based prediction models for osteoporosis in individuals under the recommended age for DXA examination. Data of 2210 men aged 50–69 and 1099 women aged 50–64 obtained from the Korea National Health and Nutrition Examination Survey IV–V were analyzed. Extreme gradient boosting (XGBoost) was used to find relevant clinical features and applied to three machine learning models: XGBoost, logistic regression, and a multilayer perceptron. For the prediction of osteoporosis, the XGBoost model using the top 20 features extracted from XGBoost showed the most reliable performance with area under the receiver operating characteristic curve (AUROC) of 0.73 and 0.79 in men and women, respectively. We compared the diagnostic accuracy of the Shapley additive explanation values based on a risk-score model obtained from XGBoost and conventional osteoporosis risk assessment tools for prediction of osteoporosis using optimal cut-off values for each model. We observed that a cut-off risk score of ≥ 28 in men and ≥ 47 in women was optimal to classify a positive screening for osteoporosis (an AUROC of 0.86 in men and 0.91 in women). The XGBoost-based osteoporosis-prediction model outperformed conventional risk assessment tools. Therefore, machine learning-based prediction models are a more suitable option than conventional risk assessment methods for screening osteoporosis in individuals under the recommended age for DXA examination.



Similar content being viewed by others
References
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936. https://doi.org/10.1016/s0140-6736(02)08761-5
Kanis J, Glüer C-C (2000) An update on the diagnosis and assessment of osteoporosis with densitometry. Osteoporos Int 11:192–202
Ahn SH, Park SM, Park SY et al (2020) Osteoporosis and osteoporotic fracture fact sheet in Korea. J Bone Metab 27(4):281–290. https://doi.org/10.11005/jbm.2020.27.4.281
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, National Osteoporosis F (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381. https://doi.org/10.1007/s00198-014-2794-2
Obermeyer Z, Emanuel EJ (2016) Predicting the future—big data, machine learning, and clinical medicine. N Engl J Med 375:1216–1219. https://doi.org/10.1056/NEJMp1606181
Cruz AS, Lins HC, Medeiros RVA, Filho JMF, da Silva SG (2018) Artificial intelligence on the identification of risk groups for osteoporosis, a general review. Biomed Eng Online 17:12. https://doi.org/10.1186/s12938-018-0436-1
Kong SH, Ahn D, Kim BR, Srinivasan K, Ram S, Kim H, Hong AR, Kim JH, Cho NH, Shin CS (2020) A novel fracture prediction model using machine learning in a community-based cohort. JBMR Plus 4:e10337. https://doi.org/10.1002/jbm4.10337
Lee S, Choe EK, Kang HY, Yoon JW, Kim HS (2020) The exploration of feature extraction and machine learning for predicting bone density from simple spine X-ray images in a Korean population. Skelet Radiol 49:613–618. https://doi.org/10.1007/s00256-019-03342-6
Su Y, Kwok TCY, Cummings SR, Yip BHK, Cawthon PM (2019) Can Classification and regression tree analysis help identify clinically meaningful risk groups for hip fracture prediction in older American men (the mros cohort study)? JBMR Plus 3:e10207. https://doi.org/10.1002/jbm4.10207
Villamor E, Monserrat C, Del Rio L, Romero-Martin JA, Ruperez MJ (2020) Prediction of osteoporotic hip fracture in postmenopausal women through patient-specific FE analyses and machine learning. Comput Methods Programs Biomed 193:105484. https://doi.org/10.1016/j.cmpb.2020.105484
Yoo TK, Kim SK, Kim DW, Choi JY, Lee WH, Oh E, Park EC (2013) Osteoporosis risk prediction for bone mineral density assessment of postmenopausal women using machine learning. Yonsei Med J 54:1321–1330. https://doi.org/10.3349/ymj.2013.54.6.1321
Hong AR, Kim JH, Lee JH, Kim SW, Shin CS (2019) Metabolic characteristics of subjects with spine-femur bone mineral density discordances: the Korean National Health and Nutrition Examination Survey (KNHANES 2008–2011). J Bone Miner Metab 37:835–843. https://doi.org/10.1007/s00774-018-0980-6
Rural Development Administration (2006) Food composition table, 7th edn. National Rural Resources Development Institute, Suwon
Schoeller DA, Tylavsky FA, Baer DJ et al (2005) QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr 81:1018–1025
Kim YJ, Jeon JY, Han SJ, Kim HJ, Lee KW, Kim DJ (2015) Effect of socio-economic status on the prevalence of diabetes. Yonsei Med J 56:641–647. https://doi.org/10.3349/ymj.2015.56.3.641
Chen T, Guestrin C (2016) Xgboost: A scalable tree boosting system. Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining, pp 785–794
Cox DR (1958) The regression analysis of binary sequences. J Roy Stat Soc 20:215–232
Svozil D, Kvasnicka V, Pospichal J (1997) Introduction to multi-layer feed-forward neural networks. Chemom Intell Lab Syst 39:43–62
Courtiol P, Maussion C, Moarii M et al (2019) Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat Med 25:1519–1525. https://doi.org/10.1038/s41591-019-0583-3
Desai RJ, Wang SV, Vaduganathan M, Evers T, Schneeweiss S (2020) Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA Netw Open 3:e1918962. https://doi.org/10.1001/jamanetworkopen.2019.18962
Lo-Ciganic WH, Huang JL, Zhang HH et al (2019) Evaluation of machine-learning algorithms for predicting opioid overdose risk among medicare beneficiaries with opioid prescriptions. JAMA Netw Open 2:e190968. https://doi.org/10.1001/jamanetworkopen.2019.0968
Mortazavi BJ, Bucholz EM, Desai NR, Huang C, Curtis JP, Masoudi FA, Shaw RE, Negahban SN, Krumholz HM (2019) Comparison of machine learning methods with National cardiovascular data registry models for prediction of risk of bleeding after percutaneous coronary intervention. JAMA Netw Open 2:e196835. https://doi.org/10.1001/jamanetworkopen.2019.6835
Hu H, Wang H, Wang F, Langley D, Avram A, Liu M (2018) Prediction of influenza-like illness based on the improved artificial tree algorithm and artificial neural network. Sci Rep 8:4895. https://doi.org/10.1038/s41598-018-23075-1
Hecht-Nielsen R (1989) Theory of the backpropagation neural network. Int Jt Conf Neural Netw 2:593–605
Lundberg SM (2017) LS-I A unified approach to interpreting model predictions. Proc Adv Neural Inf Process Syst 30:4768–4777
Paratz ED, Katz B (2011) Ageing holocaust survivors in Australia. Med J Aust 194:194–197
Raisz LG (2005) Screening for osteoporosis. N Engl J Med 353:164–171. https://doi.org/10.1056/NEJMcp042092
Koh LK, Sedrine WB, Torralba TP et al (2001) A simple tool to identify asian women at increased risk of osteoporosis. Osteoporos Int 12:699–705. https://doi.org/10.1007/s001980170070
Rud B, Hilden J, Hyldstrup L, Hrobjartsson A (2009) The Osteoporosis self-assessment tool versus alternative tests for selecting postmenopausal women for bone mineral density assessment: a comparative systematic review of accuracy. Osteoporos Int 20:599–607. https://doi.org/10.1007/s00198-008-0713-0
Fluss R, Faraggi D, Reiser B (2005) Estimation of the youden index and its associated cutoff point. Biom J 47:458–472. https://doi.org/10.1002/bimj.200410135
Iliou T, Anagnostopoulos CN, Anastassopoulos G (2014) Osteoporosis detection using machine learning techniques and feature selection. Int J Artif Intell Tools 23:1450014. https://doi.org/10.1142/s0218213014500146
Chiu JS, Li YC, Yu FC, Wang YF (2006) Applying an artificial neural network to predict osteoporosis in the elderly. Stud Health Technol Inform 124:609–614
Smets J, Shevroja E, Hügle T, Leslie WD, Hans D (2021) Machine learning solutions for osteoporosis-a review. J Bone Miner Res 36:833–851. https://doi.org/10.1002/jbmr.4292
Jiang X, Good LE, Spinka R, Schnatz PF (2016) Osteoporosis screening in postmenopausal women aged 50–64 years: BMI alone compared with current screening tools. Maturitas 83:59–64. https://doi.org/10.1016/j.maturitas.2015.09.009
Rizzoli R, Bonjour JP (2004) Dietary protein and bone health. J Bone Miner Res 19:527–531. https://doi.org/10.1359/jbmr.040204
Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP (1999) Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 69:727–736. https://doi.org/10.1093/ajcn/69.4.727
Hyun TH, Barrett-Connor E, Milne DB (2004) Zinc intakes and plasma concentrations in men with osteoporosis: the Rancho Bernardo Study. Am J Clin Nutr 80:715–721. https://doi.org/10.1093/ajcn/80.3.715
El-Gabalawy R, Blaney C, Tsai J, Sumner JA, Pietrzak RH (2018) Physical health conditions associated with full and subthreshold PTSD in U.S. military veterans: results from the National Health and resilience in veterans study. J Affect Disord 227:849–853. https://doi.org/10.1016/j.jad.2017.11.058
Ochs-Balcom HM, Hovey KM, Andrews C et al (2020) Short sleep is associated with low bone mineral density and osteoporosis in the women’s health initiative. J Bone Miner Res 35:261–268. https://doi.org/10.1002/jbmr.3879
Lucassen EA, de Mutsert R, le Cessie S, Appelman-Dijkstra NM, Rosendaal FR, van Heemst D, den Heijer M, Biermasz NR (2017) Poor sleep quality and later sleep timing are risk factors for osteopenia and sarcopenia in middle-aged men and women: the NEO study. PLoS ONE 12:e0176685. https://doi.org/10.1371/journal.pone.0176685
Brennan SL, Pasco JA, Urquhart DM, Oldenburg B, Hanna F, Wluka AE (2009) The association between socioeconomic status and osteoporotic fracture in population-based adults: a systematic review. Osteoporos Int 20:1487–1497. https://doi.org/10.1007/s00198-008-0822-9
Funding
This research was supported by the National Cancer Center (NCC-2010020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Hyun Woo Park, Hyojung Jung, Kyoung Yeon Back, Hyeon Ju Choi, Kwang Sun Ryu, Hyo Soung Cha, Eun Kyung Lee, A Ram Hong, and Yul Hwangbo declare that they have no conflicts of interest.
Human and Animal Rights
The study was conducted in accordance with the Declaration of Helsinki. The KNHANES surveys were reviewed and approved by the Institutional Review Board of the Korea Center for Disease Control and Prevention (IRB Nos. 2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21, and 2011-02CON06-C).
Informed Consent
All participants provided written informed consents prior to survey enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 439 kb)
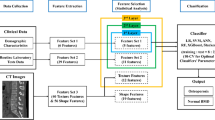
An overview of the study flow for developing osteoporosis-prediction models. XGBoost, extreme gradient boosting; MLP, multilayer perceptron.
Supplementary file3 (TIF 1284 kb)
Correlation matrix of the extracted 20 features in men (a) and women (b).
Supplementary file5 (TIF 1126 kb)
Performances in AUROC and AUPRC using XGBoost model with top-20 importance features in men (a–b) and women (c–d). AUROC, area under the receiver operating characteristics curve; AUPRC, area under the precision-recall curve.
Rights and permissions
About this article
Cite this article
Park, H.W., Jung, H., Back, K.Y. et al. Application of Machine Learning to Identify Clinically Meaningful Risk Group for Osteoporosis in Individuals Under the Recommended Age for Dual-Energy X-Ray Absorptiometry. Calcif Tissue Int 109, 645–655 (2021). https://doi.org/10.1007/s00223-021-00880-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00880-x