Abstract
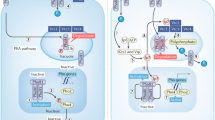
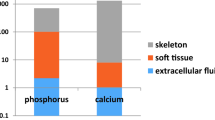
Phosphorus plays a vital role in diverse biological processes including intracellular signaling, membrane integrity, and skeletal biomineralization; therefore, the regulation of phosphorus homeostasis is essential to the well-being of the organism. Cells and whole organisms respond to changes in inorganic phosphorus (Pi) concentrations in their environment by adjusting Pi uptake and altering biochemical processes in cells (local effects) and distant organs (endocrine effects). Unicellular organisms, such as bacteria and yeast, express specific Pi-binding proteins on the plasma membrane that respond to changes in ambient Pi availability and transduce intracellular signals that regulate the expression of genes involved in cellular Pi uptake. Multicellular organisms, including humans, respond at a cellular level to adapt to changes in extracellular Pi concentrations and also have endocrine pathways which integrate signals from various organs (e.g., intestine, kidneys, parathyroid glands, bone) to regulate serum Pi concentrations and whole-body phosphorus balance. In mammals, alterations in the concentrations of extracellular Pi modulate type III sodium–phosphate cotransporter activity on the plasma membrane, and trigger changes in cellular function. In addition, elevated extracellular Pi induces activation of fibroblast growth factor receptor, Raf/mitogen-activated protein kinase/ERK kinase (MEK)/extracellular signal-regulated kinase (ERK) and Akt pathways, which modulate gene expression in various mammalian cell types. Excessive Pi exposure, especially in patients with chronic kidney disease, leads to endothelial dysfunction, accelerated vascular calcification, and impaired insulin secretion.





Similar content being viewed by others
References
Chakraborty A, Kim S, Snyder SH (2011) Inositol pyrophosphates as mammalian cell signals. Sci Signal 4:re1
Angelova PR, Baev AY, Berezhnov AV, Abramov AY (2016) Role of inorganic polyphosphate in mammalian cells: from signal transduction and mitochondrial metabolism to cell death. Biochem Soc Trans. 44:40–45
Amanzadeh J, Reilly RF (2006) Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract. 2:136–148
Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ (2013) Phosphorus, phosphorous, and phosphate. Hemodial Int. 17:479–482
Mouillon J-M, Persson BL (2006) New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS Yeast Res. 6:171–176
Virkki LV, Biber J, Murer H, Forster IC (2007) Phosphate transporters: a tale of two solute carrier families. Am J Physiol. 293:F643–654
Michigami T, Kawai M, Yamazaki M, Ozono K (2018) Phosphate as a signaling molecule and its sensing mechanism. Physiol Rev. 98:2317–2348
Bon N, Couasnay G, Bourgine A, Sourice S, Beck-Cormier S, Guicheux J et al (2018) Phosphate (Pi)-regulated heterodimerization of the high-affinity sodium-dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J Biol Chem. 293:2102–2114
Polgreen KE, Kemp GJ, Leighton B, Radda GK (1994) Modulation of Pi transport in skeletal muscle by insulin and IGF-1. Biochim Biophys Acta. 1223:279–284
Suzuki A, Palmer G, Bonjour JP, Caverzasio J (2001) Stimulation of sodium-dependent inorganic phosphate transport by activation of Gi/o-protein-coupled receptors by epinephrine in MC3T3-E1 osteoblast-like cells. Bone 28:589–594
Ferreira GC, Pedersen PL (1993) Phosphate transport in mitochondria: past accomplishments, present problems, and future challenges. J Bioenerg Biomembr. 25:483–492
Pisoni RL (1991) Characterization of a phosphate transport system in human fibroblast lysosomes. J Biol Chem. 266:979–985
Burchell A (1996) Endoplasmic reticulum phosphate transport. Kidney Int. 49:953–958
Brautbar N, Leibovici H, Massry SG (1983) On the mechanism of hypophosphatemia during acute hyperventilation: evidence for increased muscle glycolysis. Miner Electrolyte Metab. 9:45–50
Peters J, Binswanger U (1988) Calcium and inorganic phosphate secretion of rat ileum in vitro. Influence of uremia and 1,25 (OH)2D3 inhibition. Res Exp Med (Berl). 188:139–149
Bergwitz C, Jüppner H (2010) Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 61:91–104
Lamarche MG, Wanner BL, Crépin S, Harel J (2008) The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev. 32:461–473
Levi M, Gratton E, Forster IC, Hernando N, Wagner CA, Biber J et al (2019) Mechanisms of phosphate transport. Nat Rev Nephrol. 15:482–500
Kimata M, Michigami T, Tachikawa K, Okada T, Koshimizu T, Yamazaki M et al (2010) Signaling of extracellular inorganic phosphate up-regulates cyclin D1 expression in proliferating chondrocytes via the Na+/Pi cotransporter Pit-1 and Raf/MEK/ERK pathway. Bone 47:938–947
Bon N, Frangi G, Sourice S, Guicheux J, Beck-Cormier S, Beck L (2018) Phosphate-dependent FGF23 secretion is modulated by PiT2/Slc20a2. Mol Metab. 11:197–204
Li X, Yang H-Y, Giachelli CM (2006) Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 98:905–912
Beck L, Leroy C, Salaün C, Margall-Ducos G, Desdouets C, Friedlander G (2009) Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity. J Biol Chem. 284:31363–31374
Knöpfel T, Himmerkus N, Günzel D, Bleich M, Hernando N, Wagner CA (2019) Paracellular transport of phosphate along the intestine. Am J Physiol. 317(2):G233
Amasheh S, Fromm M, Günzel D (2011) Claudins of intestine and nephron—a correlation of molecular tight junction structure and barrier function. Acta Physiol. 201:133–140
Garcia-Hernandez V, Quiros M, Nusrat A (2017) Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 1397:66–79
Marks J, Debnam ES, Unwin RJ (2010) Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol. 299:F285–296
Bai L, Collins JF, Ghishan FK (2000) Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am J Physiol Cell Physiol. 279:C1135–1143
Olah Z, Lehel C, Anderson WB, Eiden MV, Wilson CA (1994) The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J Biol Chem. 269:25426–25431
Christakos S, Lieben L, Masuyama R, Carmeliet G (2014) Vitamin D endocrine system and the intestine. BoneKEy Rep. 3:496
Xu H, Bai L, Collins JF, Ghishan FK (2002) Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)(2) vitamin D(3). Am J Physiol Cell Physiol. 282:C487–493
Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y et al (2004) Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol. 287:F39–47
Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K (2003) Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol. 285:F1271–1278
Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS (1998) Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95:5372–5377
Trepiccione F, Capasso G (2011) SGK3: a novel regulator of renal phosphate transport? Kidney Int. 80:13–15
Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ et al (2007) Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA 104:11085–11090
Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I et al (2009) Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol. 20:104–113
Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H et al (2009) The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol. 296:F691–699
Villa-Bellosta R, Sorribas V (2010) Compensatory regulation of the sodium/phosphate cotransporters NaPi-IIc (SCL34A3) and Pit-2 (SLC20A2) during Pi deprivation and acidosis. Pflugers Arch. 459:499–508
Giovannini D, Touhami J, Charnet P, Sitbon M, Battini J-L (2013) Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep. 3:1866–1873
Ansermet C, Moor MB, Centeno G, Auberson M, Hu DZ, Baron R et al (2017) Renal fanconi syndrome and hypophosphatemic rickets in the absence of xenotropic and polytropic retroviral receptor in the nephron. J Am Soc Nephrol. 28:1073–1078
Legati A, Giovannini D, Nicolas G, López-Sánchez U, Quintáns B, Oliveira JRM et al (2015) Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat Genet. 47:579–581
Farrow EG, White KE (2010) Recent advances in renal phosphate handling. Nat Rev Nephrol. 6:207–217
Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA (2006) The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 69:495–503
Breusegem SY, Takahashi H, Giral-Arnal H, Wang X, Jiang T, Verlander JW et al (2009) Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Renal Physiol. 297:F350–361
Murer H, Hernando N, Forster I, Biber J (2003) Regulation of Na/Pi transporter in the proximal tubule. Annu Rev Physiol. 65:531–542
Forte LR (2003) A novel role for uroguanylin in the regulation of sodium balance. J Clin Invest. 112:1138–1141
Lee FN, Oh G, McDonough AA, Youn JH (2007) Evidence for gut factor in K+ homeostasis. Am J Physiol Renal Physiol. 293:F541–547
Conigrave AD, Brown EM (2006) Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol. 291:G753–761
Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T et al (2006) Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 70:2141–2147
Ritthaler T, Traebert M, Lötscher M, Biber J, Murer H, Kaissling B (1999) Effects of phosphate intake on distribution of type II Na/Pi cotransporter mRNA in rat kidney. Kidney Int. 55:976–983
Scanni R, vonRotz M, Jehle S, Hulter HN, Krapf R (2014) The human response to acute enteral and parenteral phosphate loads. J Am Soc Nephrol. 25:2730–2739
Thomas L, Bettoni C, Knöpfel T, Hernando N, Biber J, Wagner CA (2017) Acute adaption to oral or intravenous phosphate requires parathyroid hormone. J Am Soc Nephrol. 28:903–914
Kido S, Miyamoto K, Mizobuchi H, Taketani Y, Ohkido I, Ogawa N et al (1999) Identification of regulatory sequences and binding proteins in the type II sodium/phosphate cotransporter NPT2 gene responsive to dietary phosphate. J Biol Chem. 274:28256–28263
Beck L, Tenenhouse HS, Meyer RA, Meyer MH, Biber J, Murer H (1996) Renal expression of Na+-phosphate cotransporter mRNA and protein: effect of the Gy mutation and low phosphate diet. Pflugers Arch. 431:936–941
Hoag HM, Martel J, Gauthier C, Tenenhouse HS (1999) Effects of Npt2 gene ablation and low-phosphate diet on renal Na(+)/phosphate cotransport and cotransporter gene expression. J Clin Invest. 104:679–686
Kilav R, Silver J, Biber J, Murer H, Naveh-Many T (1995) Coordinate regulation of rat renal parathyroid hormone receptor mRNA and Na-Pi cotransporter mRNA and protein. Am J Physiol. 268:F1017–1022
Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T et al (2007) Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 25:419–422
Ferrari SL, Bonjour J-P, Rizzoli R (2005) Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 90:1519–1524
Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB (2003) Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 64:2272–2279
Zhang S, Gillihan R, He N, Fields T, Liu S, Green T et al (2013) Dietary phosphate restriction suppresses phosphaturia but does not prevent FGF23 elevation in a mouse model of chronic kidney disease. Kidney Int. 84:713–721
Haussler M, Hughes M, Baylink D, Littledike ET, Cork D, Pitt M (1977) Influence of phosphate depletion on the biosynthesis and circulating level of 1alpha, 25-dihydroxyvitamin D. Adv Exp Med Biol. 81:233–250
Tanaka Y, Deluca HF (1973) The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 154:566–574
Tenenhouse HS, Martel J, Gauthier C, Zhang MY, Portale AA (2001) Renal expression of the sodium/phosphate cotransporter gene, Npt2, is not required for regulation of renal 1 alpha-hydroxylase by phosphate. Endocrinology 142:1124–1129
Yamazaki M, Ozono K, Okada T, Tachikawa K, Kondou H, Ohata Y et al (2010) Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells. J Cell Biochem. 111:1210–1221
Conrads KA, Yi M, Simpson KA, Lucas DA, Camalier CE, Yu L-R et al (2005) A combined proteome and microarray investigation of inorganic phosphate-induced pre-osteoblast cells. Mol Cell Proteomics. 4:1284–1296
Julien M, Magne D, Masson M, Rolli-Derkinderen M, Chassande O, Cario-Toumaniantz C et al (2007) Phosphate stimulates matrix Gla protein expression in chondrocytes through the extracellular signal regulated kinase signaling pathway. Endocrinology 148:530–537
Nishino J, Yamazaki M, Kawai M, Tachikawa K, Yamamoto K, Miyagawa K et al (2017) Extracellular phosphate induces the expression of dentin matrix protein 1 through the FGF receptor in osteoblasts. J Cell Biochem. 118:1151–1163
Camalier CE, Yi M, Yu L-R, Hood BL, Conrads KA, Lee YJ et al (2013) An integrated understanding of the physiological response to elevated extracellular phosphate. J Cell Physiol. 228:1536–1550
Kawai M, Kinoshita S, Ozono K, Michigami T (2016) Inorganic phosphate activates the AKT/mTORC1 pathway and shortens the life span of an α-Klotho-deficient model. J Am Soc Nephrol. 27:2810–2824
Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A et al (1996) Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res. 11:970–976
Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez Cruz L et al (1998) High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol. 9:1845–1852
Almadén Y, Canalejo A, Ballesteros E, Añón G, Rodríguez M (2000) Effect of high extracellular phosphate concentration on arachidonic acid production by parathyroid tissue in vitro. J Am Soc Nephrol. 11:1712–1718
Dusso AS, Pavlopoulos T, Naumovich L, Lu Y, Finch J, Brown AJ et al (2001) p21(WAF1) and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth. Kidney Int. 59:855–865
Tatsumi S, Segawa H, Morita K, Haga H, Kouda T, Yamamoto H et al (1998) Molecular cloning and hormonal regulation of PiT-1, a sodium-dependent phosphate cotransporter from rat parathyroid glands. Endocrinology 139:1692–1699
Geng Y, Mosyak L, Kurinov I, Zuo H, Sturchler E, Cheng TC, et al (2016) Structural mechanism of ligand activation in human calcium-sensing receptor. eLife. https://doi.org/10.7554/eLife.13662
Moallem E, Kilav R, Silver J, Naveh-Many T (1998) RNA-Protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J Biol Chem. 273:5253–5259
Nechama M, Ben-Dov IZ, Briata P, Gherzi R, Naveh-Many T (2008) The mRNA decay promoting factor K-homology splicing regulator protein post-transcriptionally determines parathyroid hormone mRNA levels. FASEB J. 22:3458–3468
Dinur M, Kilav R, Sela-Brown A, Jacquemin-Sablon H, Naveh-Many T (2006) In vitro evidence that upstream of N-ras participates in the regulation of parathyroid hormone messenger ribonucleic acid stability. Mol Endocrinol Baltim Md. 20:1652–1660
Nechama M, Uchida T, Mor Yosef-Levi I, Silver J, Naveh-Many T (2009) The peptidyl-prolyl isomerase Pin1 determines parathyroid hormone mRNA levels and stability in rat models of secondary hyperparathyroidism. J Clin Invest. 119:3102–3114
Hu MC, Shiizaki K, Kuro-o M, Moe OW (2013) Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 75:503–533
Perwad F, Azam N, Zhang MYH, Yamashita T, Tenenhouse HS, Portale AA (2005) Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146:5358–5364
Takashi Y, Kosako H, Sawatsubashi S, Kinoshita Y, Ito N, Tsoumpra MK et al (2019) Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc Natl Acad Sci USA 116:11418–11427
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G (2016) Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 96:365–408
Bikle DD (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 21:319–329
Condamine L, Menaa C, Vrtovsnik F, Vztovsnik F, Friedlander G, Garabédian M (1994) Local action of phosphate depletion and insulin-like growth factor 1 on in vitro production of 1,25-dihydroxyvitamin D by cultured mammalian kidney cells. J Clin Invest. 94:1673–1679
Fukase M, Birge SJ, Rifas L, Avioli LV, Chase LR (1982) Regulation of 25 hydroxyvitamin D3 1-hydroxylase in serum-free monolayer culture of mouse kidney. Endocrinology 110:1073–1075
Gray RW (1981) Control of plasma 1,25-(OH)2-vitamin D concentrations by calcium and phosphorus in the rat: effects of hypophysectomy. Calcif Tissue Int. 33:485–488
Halloran BP, Spencer EM (1988) Dietary phosphorus and 1,25-dihydroxyvitamin D metabolism: influence of insulin-like growth factor I. Endocrinology 123:1225–1229
Nguyen TT, Quan X, Xu S, Das R, Cha S-K, Kong ID et al (2016) Intracellular alkalinization by phosphate uptake via type III sodium-phosphate cotransporter participates in high-phosphate-induced mitochondrial oxidative stress and defective insulin secretion. FASEB J. 30:3979–3988
Nguyen TT, Quan X, Hwang K-H, Xu S, Das R, Choi S-K et al (2015) Mitochondrial oxidative stress mediates high-phosphate-induced secretory defects and apoptosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 308:E933–941
Kovesdy CP, Sharma K, Kalantar-Zadeh K (2008) Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis. 52:766–777
Williams ME, Garg R (2014) Glycemic management in ESRD and earlier stages of CKD. Am J Kidney Dis. 63:S22–38
Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M et al (2009) Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 20:1504–1512
Stevens KK, Denby L, Patel RK, Mark PB, Kettlewell S, Smith GL et al (2017) Deleterious effects of phosphate on vascular and endothelial function via disruption to the nitric oxide pathway. Nephrol Dial Transplant. 32:1617–1627
Peng A, Wu T, Zeng C, Rakheja D, Zhu J, Ye T et al (2011) Adverse effects of simulated hyper- and hypo-phosphatemia on endothelial cell function and viability. PLoS ONE 6:e23268
Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN (2000) Abnormalities of endothelial function in patients with predialysis renal failure. Heart 83:205–209
Van TV, Watari E, Taketani Y, Kitamura T, Shiota A, Tanaka T et al (2012) Dietary phosphate restriction ameliorates endothelial dysfunction in adenine-induced kidney disease rats. J Clin Biochem Nutr. 51:27–32
Annuk M, Soveri I, Zilmer M, Lind L, Hulthe J, Fellström B (2005) Endothelial function, CRP and oxidative stress in chronic kidney disease. J Nephrol. 18:721–726
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D et al (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 342:1478–1483
Mizobuchi M, Towler D, Slatopolsky E (2009) Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 20:1453–1464
London GM (2003) Cardiovascular calcifications in uremic patients: clinical impact on cardiovascular function. J Am Soc Nephrol. 14:S305–309
Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM (2011) Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 109:697–711
Schlieper G, Schurgers L, Brandenburg V, Reutelingsperger C, Floege J (2016) Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transplant. 31:31–39
Paloian NJ, Giachelli CM (2014) A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol. 307:F891–900
Giachelli CM (2003) Vascular calcification: in vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol. 14:S300–304
Cancela AL, Santos RD, Titan SM, Goldenstein PT, Rochitte CE, Lemos PA et al (2012) Phosphorus is associated with coronary artery disease in patients with preserved renal function. PLoS ONE 7:e36883
Vervloet M, Cozzolino M (2017) Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 91:808–817
Jablonski KL, Chonchol M (2013) Vascular calcification in end-stage renal disease. Hemodial Int Int Symp Home Hemodial. 17(Suppl 1):S17–21
Benz K, Hilgers K-F, Daniel C, Amann K (2018) Vascular calcification in chronic kidney disease: the role of inflammation. Int J Nephrol. 2018:4310379
Holden RM, Hétu M-F, Li TY, Ward E, Couture LE, Herr JE et al (2019) The heart and kidney: abnormal phosphate homeostasis is associated with atherosclerosis. J Endocr Soc. 3:159–170
Bernelot Moens SJ, Verweij SL, van der Valk FM, van Capelleveen JC, Kroon J, Versloot M et al (2017) Arterial and cellular inflammation in patients with CKD. J Am Soc Nephrol JASN. 28:1278–1285
Voelkl J, Cejka D, Alesutan I (2019) An overview of the mechanisms in vascular calcification during chronic kidney disease. Curr Opin Nephrol Hypertens. 28:289–296
Cho I-J, Chang H-J, Park H-B, Heo R, Shin S, Shim CY et al (2015) Aortic calcification is associated with arterial stiffening, left ventricular hypertrophy, and diastolic dysfunction in elderly male patients with hypertension. J Hypertens. 33:1633–1641
Morita S, Asou T, Kuboyama I, Harasawa Y, Sunagawa K, Yasui H (2002) Inelastic vascular prosthesis for proximal aorta increases pulsatile arterial load and causes left ventricular hypertrophy in dogs. J Thorac Cardiovasc Surg. 124:768–774
Moody WE, Edwards NC, Chue CD, Ferro CJ, Townend JN (2013) Arterial disease in chronic kidney disease. Heart 99:365–372
Huveneers S, Daemen MJAP, Hordijk PL (2015) Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res. 116:895–908
Zhang D, Bi X, Liu Y, Huang Y, Xiong J, Xu X et al (2017) High phosphate-induced calcification of vascular smooth muscle cells is associated with the TLR4/NF-κb signaling pathway. Kidney Blood Press Res. 42:1205–1215
Alesutan I, Voelkl J, Feger M, Kratschmar DV, Castor T, Mia S et al (2017) Involvement of vascular aldosterone synthase in phosphate-induced osteogenic transformation of vascular smooth muscle cells. Sci Rep. 7:2059
Lang F, Ritz E, Voelkl J, Alesutan I (2013) Vascular calcification–is aldosterone a culprit? Nephrol Dial Transplant. 28:1080–1084
Wang P, Quan Z, Luo D, Chen W, Peng D (2019) Spironolactone dose-dependently alleviates the calcification of aortic rings cultured in hyperphosphatemic medium with or without hyperglycemia by suppressing phenotypic transition of VSMCs through downregulation of Pit-1. Mol Med Rep. 19:3622–3632
Yamada S, Leaf EM, Chia JJ, Cox TC, Speer MY, Giachelli CM (2018) PiT-2, a type III sodium-dependent phosphate transporter, protects against vascular calcification in mice with chronic kidney disease fed a high-phosphate diet. Kidney Int. 94:716–727
Chen NX, Moe SM (2015) Pathophysiology of vascular calcification. Curr Osteoporos Rep. 13:372–380
Speer MY, Li X, Hiremath PG, Giachelli CM (2010) Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J Cell Biochem. 110:935–947
Nishio Y, Dong Y, Paris M, O’Keefe RJ, Schwarz EM, Drissi H (2006) Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene 372:62–70
Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM et al (2012) Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 111:543–552
Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R et al (2001) Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 89:1147–1154
Speer MY, Yang H-Y, Brabb T, Leaf E, Look A, Lin W-L et al (2009) Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 104:733–741
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kritmetapak, K., Kumar, R. Phosphate as a Signaling Molecule. Calcif Tissue Int 108, 16–31 (2021). https://doi.org/10.1007/s00223-019-00636-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00636-8