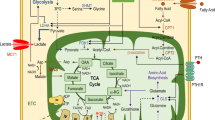
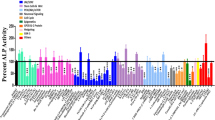
Abstract
Theobromine (THB) is one of the major xanthine-like alkaloids found in cacao plant and a variety of other foodstuffs such as tea leaves, guarana and cola nuts. Historically, THB and its derivatives have been utilized to treat cardiac and circulatory disorders, drug-induced nephrotoxicity, proteinuria and as an immune-modulator. Our previous work demonstrated that THB has the capacity to improve the formation of hydroxyl-apatite during tooth development, suggesting that it may also enhance skeletal development. With its excellent safety profile and resistance to pharmacokinetic elimination, we reasoned that it might be an excellent natural osteoanabolic supplement during pregnancy, lactation and early postnatal growth. To determine whether THB had an effect on human osteoprogenitors, we subjected primary human bone marrow mesenchymal stem cells (hMSCs) to osteogenic assays after exposure to THB in vitro and observed that THB exposure increased the rate of osteogenesis and mineralization by hMSCs. Moreover, THB exposure resulted in a list of upregulated mRNA transcripts that best matched an osteogenic tissue expression signature as compared to other tissue expression signatures archived in several databases. To determine whether oral administration of THB resulted in improved skeletal growth, we provided pregnant rats with chow supplemented with THB during pregnancy and lactation. After weaning, offspring received THB continuously until postnatal day 50 (approximately 10 mg kg−1 day−1). Administration of THB resulted in neonates with larger bones, and 50-day-old offspring accumulated greater body mass, longer and thicker femora and superior tibial trabecular parameters. The accelerated growth did not adversely affect the strength and resilience of the bones. These results indicate that THB increases the osteogenic potential of bone marrow osteoprogenitors, and dietary supplementation of a safe dose of THB to expectant mothers and during the postnatal period could accelerate skeletal development in their offspring.





Similar content being viewed by others
References
Smit HJ (2011) Theobromine and the pharmacology of cocoa. Handb Exp Pharmacol 200:201–234
Stavric B (1988) Methylxanthines: toxicity to humans. 3. Theobromine, paraxanthine and the combined effects of methylxanthines. Food Chem Toxicol 26:725–733
Champion S, Lapidus N, Cherié G, Spagnoli V, Oliary J, Solal AC (2014) Pentoxifylline in heart failure: a meta-analysis of clinical trials. Cardiovasc Ther 32:159–162
Kaur J, Sodhi GS (1992) Diuretic activity of organomercury (II) complexes of theophylline and theobromine. J Inorg Biochem 48:305–310
Skudicky D, Bergemann A, Sliwa K, Candy G, Sareli P (2001) Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensin-converting enzyme inhibitors and carvedilol: results of a randomized study. Circulation 103:1083–1088
Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P (2002) The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail 4:305–309
Nasiri-Toosi Z, Dashti-Khavidaki S, Khalili H, Lessan-Pezeshki M (2013) A review of the potential protective effects of pentoxifylline against drug-induced nephrotoxicity. Eur J Clin Pharmacol 69:1057–1073
Badri S, Dashti-Khavidaki S, Lessan-Pezeshki M, Abdollahi M (2011) A review of the potential benefits of pentoxifylline in diabetic and non-diabetic proteinuria. J Pharm Pharm Sci 14:128–137
Bell SG (2005) Immunomodulation, part I: pentoxifylline. Neonatal Netw 24:45–48
Noel C, Copin MC, Hazzan M, Labalette M, Susen S, Lelievre G, Dessaint JP (2000) Immunomodulatory effect of pentoxifylline during human allograft rejection: involvement of tumor necrosis factor-alpha and adhesion molecules. Transplantation 69:1102–1107
Costantini TW, Deree J, Peterson CY, Putnam JG, Woon T, Loomis WH, Bansal V, Coimbra R (2010) Pentoxifylline modulates p47phox activation and downregulates neutrophil oxidative burst through PKA-dependent and -independent mechanisms. Immunopharmacol Immunotoxicol 32:82–91
Rieneck K, Diamant M, Haahr PM, Schönharting M, Bendtzen K (1993) In vitro immunomodulatory effects of pentoxifylline. Immunol Lett 37:131–138
Zoumas BL, Kreiser WR, Martin RA (1980) Theobromine and caffeine content in chocolate products. J Food Sci 45:314
Craig WJ, Nguyen TT (1984) Caffeine and theobromine levels in cocoa and cocoa products. J Food Sci 49:302
Tarka SM Jr, Zoumas BL, Gans JH (1979) Short-term effects of graded levels of theobromine in laboratory rodents. Toxicol Appl Pharmacol 49:127–149
Nakamoto T, Simmons WB, Falster AU (1999) Products of apatite-forming-systems. US Patent US5919426A
Nakamoto T, Simmons WB, Falster AU (2001) Apatite-forming-systems: Methods and products. US Patent US 6183711
Nakamoto T, Falster AU, Simmons WB (2016) Theobromine: a safe and effective alternative for fluoride in dentifrices. J Caffeine Res 6(1):1–9
Amaechi BT, Porteous N, Ramalingam K, Mensinkai PK, Ccahuana Vasquez RA, Sadeghpour A, Nakamoto T (2013) Remineralization of artificial enamel lesions by theobromine. Caries Res 47:399–405
Namgung R, Tsang RC (2000) Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc Nutr Soc 59:55–63
Habbick BF, Blakley PM, Houston CS, Snyder RE, Senthilselvan A, Nanson JL (1998) Bone age and growth in fetal alcohol syndrome. Alcohol Clin Exp Res 22:1312–1316
Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, Cooper C (2001) Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res 16:1694–1703
Curtis EM, Moon RJ, Dennison EM, Harvey NC (2014) Prenatal calcium and vitamin D intake, and bone mass in later life. Curr Osteoporos Rep 12:194–204
Sanz-Salvador L, Garcia-Perez MA, Tarin JJ, Cano A (2015) Bone metabolic changes during pregnancy: a period of vulnerability to osteoporosis and fracture. Eur J Endocrinol 172:R53–R65
Gerin M, Jambon G, Fouque-Aubert A, Raybaud C, Cochat P, Claris O, Bacchetta J (2016) Neonatal transient hypophosphatemic hypercalciuric rickets in dizygous twins: a role for maternal alendronate therapy before pregnancy or antireflux medications? Arch Pediatr 23:957–962
French AE, Kaplan N, Lishner M, Koren G (2003) Taking bisphosphonates during pregnancy. Can Fam Physician 49:1281–1282
Rosenkranz HS, Ennever FK (1987) Evaluation of the genotoxicity of theobromine and caffeine. Food Chem Toxicol 25:247–251
Shively CA, White DM, Blauch JL, Tarka SM Jr (1984) Dominant lethal testing of theobromine in rats. Toxicol Lett 20:325–329
Drouillard DD, Vesell ES, Dvorchik BH (1978) Studies on theobromine disposition in normal subjects. Alterations induced by dietary abstention from or exposure to methylxanthines. Clin Pharmacol Ther 23:296–302
Shively CA, Tarka SM Jr, Arnaud MJ, Dvorchik BH, Passananti GT, Vesell ES (1985) High levels of methylxanthines in chocolate do not alter theobromine disposition. Clin Pharmacol Ther 37:415–424
Gregory CA, Prockop DJ (2007) Fundementals of culture and characterization of mesenchymal stem/progenitor cells from bone marrow stroma. In: Freshney RI, Stacey GN, Auerbach JM (eds) Culture of human stem cells. Wiley-Liss, Hoboken, p 208
Zeitouni S, Krause U, Clough BH, Halderman H, Falster A, Blalock DT, Chaput CD, Sampson HW, Gregory CA (2012) Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci Transl Med 4:132ra155
Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA (2003) DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4:P3
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Krause U, Seckinger A, Gregory CA (2011) Assays of osteogenic differentiation by cultured human mesenchymal stem cells. Methods Mol Biol 698:215–230
Johnsson MS, Nancollas GH (1992) The role of brushite and octacalcium phosphate in apatite formation. Crit Rev Oral Biol Med 3:61–82
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280:33132–33140
Dong YF, Soung DY, Schwarz EM, O’Keefe RJ, Drissi H (2006) Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol 208:77–86
Zhang R, Oyajobi BO, Harris SE, Chen D, Tsao C, Deng HW, Zhao M (2013) Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 52:145–156
Kugimiya F, Kawaguchi H, Ohba S, Kawamura N, Hirata M, Chikuda H, Azuma Y, Woodgett JR, Nakamura K, Chung UI (2007) GSK-3beta controls osteogenesis through regulating Runx2 activity. PLoS ONE 2:e837
Wang Z, Zhang JN, Hu XF, Chen XL, Wang XR, Zhao TT, Peng MJ, Zou P (2010) Effects of pentoxifylline on Wnt/beta-catenin signaling in mice chronically exposed to cigarette smoke. Chin Med J 123:2688–2694
Jang YJ, Koo HJ, Sohn EH, Kang SC, Rhee DK, Pyo S (2015) Theobromine inhibits differentiation of 3T3-L1 cells during the early stage of adipogenesis via AMPK and MAPK signaling pathways. Food Funct 6:2365–2374
Krause U, Harris S, Green A, Ylostalo J, Zeitouni S, Lee N, Gregory CA (2010) Pharmaceutical modulation of canonical Wnt signaling in multipotent stromal cells for improved osteoinductive therapy. Proc Natl Acad Sci USA 107:4147–4152
Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA (2002) Regulation of Wnt signaling during adipogenesis. J Biol Chem 277:30998–31004
Farmer SR (2005) Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond) 29(Suppl 1):S13–S16
Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR (2003) Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J 376:607–613
Xu C, Wang J, Zhu T, Shen Y, Tang X, Fang L, Xu Y (2016) Cross-talking between PPAR and WNT signaling and its regulation in mesenchymal stem cell differentiation. Curr Stem Cell Res Ther 11(3):247–254
Takada I, Kouzmenko AP, Kato S (2009) Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 5:442–447
Tarka SM Jr, Applebaum RS, Borzelleca JF (1986) Evaluation of the perinatal, postnatal and teratogenic effects of cocoa powder and theobromine in Sprague-Dawley/CD rats. Food Chem Toxicol 24:375–382
Inoue H, Kobayashi-Hattori K, Horiuchi Y, Oishi Y, Arai S, Takita T (2006) Regulation of the body fat percentage in developmental-stage rats by methylxanthine derivatives in a high-fat diet. Biosci Biotechnol Biochem 70:1134–1139
Parfitt AM (1994) The two faces of growth: benefits and risks to bone integrity. Osteoporos Int 4:382–398
Heaney RP, Weaver CM (2005) Newer perspectives on calcium nutrition and bone quality. J Am Coll Nutr 24:574S–581S
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Seminara S, Rapisardi G, La Cauza F, Mattei P, Donzelli G (2000) Catch-up growth in short-at-birth NICU graduates. Horm Res 53:139–143
van der Steen M, Hokken-Koelega AC (2016) Growth and metabolism in children born small for gestational age. Endocrinol Metab Clin North Am 45:283–294
Acknowledgements
Funded in part by Theocorp Holding Co. LLC, the Institute for Regenerative Medicine Program Funds and NIH 2P40RR017447-07. We thank Suzanne Zeitouni for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TN is co-founder of Theocorp Holding Co. CAG and HRR are members of the Scientific Advisory Board of Theocorp Holding Co. BHC, JY, EB, EPM and TJB state no competing interests.
Human and animal rights and informed consent
Animal studies were performed in accordance with an ethical animal use protocol approved by the Texas A&M Institutional Animal Care and Use Committee. Human mesenchymal stem cells were acquired from the Texas A&M Health Science Center Institute for Regenerative Medicine in accordance with a Baylor Scott and White Hospital and Texas A&M Health Science Center Institutional Review Board approved protocol for ethical acquisition of human tissue samples.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clough, B.H., Ylostalo, J., Browder, E. et al. Theobromine Upregulates Osteogenesis by Human Mesenchymal Stem Cells In Vitro and Accelerates Bone Development in Rats. Calcif Tissue Int 100, 298–310 (2017). https://doi.org/10.1007/s00223-016-0215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0215-6