Abstract
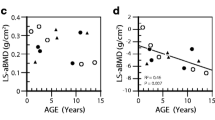
Cyclical intravenous treatment with pamidronate is widely used to treat osteogenesis imperfecta (OI) types I, III, and IV, which are due to dominant mutations affecting collagen type I alpha chains. There is no information about the effects of pamidronate in children with OI type VII, an autosomal-recessive form of OI caused by a mutation in the cartilage-associated protein gene. In this retrospective single-center study, we compared the effects of pamidronate in four girls with OI type VII (age range 3.9–12.7 years) to those in eight girls with OI types caused by collagen type I mutations who were matched for age and disease severity. During 3 years of pamidronate therapy, lumbar spine areal bone mineral density increased and lumbar vertebral bodies improved in shape in patients with OI type VII. Other outcomes such as fracture rates and mobility scores did not show statistically significant changes in this small study cohort. There were no significant side effects noted during the time of follow-up. Thus, intravenous treatment with pamidronate seems to be safe and of some benefit in patients with OI type VII.


Similar content being viewed by others
References
Ward LM, Rauch F, Travers R, Chabot G, Azouz EM, Lalic L, Roughley PJ, Glorieux FH (2002) Osteogenesis imperfecta type VII: an autosomal recessive form of brittle bone disease. Bone 31:12–18
Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, Monticone M, Castagnola P, Rauch F, Glorieux FH, Vranka J, Bachinger HP, Pace JM, Schwarze U, Byers PH, Weis M, Fernandes RJ, Eyre DR, Yao Z, Boyce BF, Lee B (2006) CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 127:291–304
Barnes AM, Chang W, Morello R, Cabral WA, Weis M, Eyre DR, Leikin S, Makareeva E, Kuznetsova N, Uveges TE, Ashok A, Flor AW, Mulvihill JJ, Wilson PL, Sundaram UT, Lee B, Marini JC (2006) Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med 355:2757–2764
Rauch F, Glorieux FH (2004) Osteogenesis imperfecta. Lancet 363:1377–1385
D’Amour P, Labelle F, Lecavalier L, Plourde V, Harvey D (1986) Influence of serum Ca concentration on circulating molecular forms of PTH in three species. Am J Physiol 251:E680–E687
Bollen AM, Eyre DR (1994) Bone resorption rates in children monitored by the urinary assay of collagen type I cross-linked peptides. Bone 15:31–34
Land C, Rauch F, Munns CF, Sahebjam S, Glorieux FH (2006) Vertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatment. Bone 39:901–906
Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ 3rd (1991) Classification of vertebral fractures. J Bone Miner Res 6:207–215
Salle BL, Braillon P, Glorieux FH, Brunet J, Cavero E, Meunier PJ (1992) Lumbar bone mineral content measured by dual energy X-ray absorptiometry in newborns and infants. Acta Paediatr 81:953–958
Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL (2002) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60
Rauch F, Neu CM, Wassmer G, Beck B, Rieger-Wettengl G, Rietschel E, Manz F, Schoenau E (2002) Muscle analysis by measurement of maximal isometric grip force: new reference data and clinical applications in pediatrics. Pediatr Res 51:505–510
Bleck EE (1981) Nonoperative treatment of osteogenesis imperfecta: orthotic and mobility management. Clin Orthop Relat Res 159:111–122
Haley SM, Coster WJ, Ludlow LH, Haltiwanger JT, Anrellos PJ (1992) Pediatric Evaluation of Disability Inventory (PEDI): development, standardization and administrative manual, New England Medical Center, Boston
Glorieux FH, Travers R, Taylor A, Bowen JR, Rauch F, Norman M, Parfitt AM (2000) Normative data for iliac bone histomorphometry in growing children. Bone 26:103–109
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R (1998) Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med 339:947–952
Rauch F, Plotkin H, Travers R, Zeitlin L, Glorieux FH (2003) Osteogenesis imperfecta types I, III, and IV: effect of pamidronate therapy on bone and mineral metabolism. J Clin Endocrinol Metab 88:986–992
Land C, Rauch F, Travers R, Glorieux FH (2007) Osteogenesis imperfecta type VI in childhood and adolescence: effects of cyclical intravenous pamidronate treatment. Bone 40:638–644
Zeitlin L, Rauch F, Travers R, Munns C, Glorieux FH (2006) The effect of cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta type V. Bone 38:13–20
Rauch F, Travers R, Plotkin H, Glorieux FH (2002) The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest 110:1293–1299
Acknowledgments
We thank Mark Lepik for the preparation of the figures. This study was supported by the Shriners of North America. F.R. is a Chercheur-Boursier Clinicien of the Fonds de la Recherche en Santé du Québec.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheung, M.S., Glorieux, F.H. & Rauch, F. Intravenous Pamidronate in Osteogenesis Imperfecta Type VII. Calcif Tissue Int 84, 203–209 (2009). https://doi.org/10.1007/s00223-008-9211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-008-9211-9