Abstract
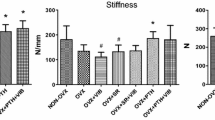
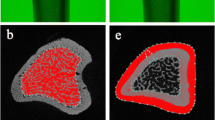
This study was designed to determine the modulatory effect of estrogen on mechanical stimulation in bone. Trabecular and cortical bone compartments of ovariectomized rats exposed to whole-body vibration of different amplitudes were evaluated by peripheral quantitative computed tomographic (pQCT) analysis and histomorphometry and compared to controls not exposed to vibration. Rats underwent whole-body vibration (20 minutes/day, 5 days/week) on a vibration platform for 2 months. The control rats were placed on the platform without vibration for the same time. We divided rats into six groups: a sham control (SHAM); a sham vibrated (SHAM-V) at 30 Hz, 0.6 g; a SHAM-V at 30 Hz, 3g; an ovariectomized control (OVX); an ovariectomized vibrated (OVX-V) at 30 Hz, 0.6 g; and an OVX-V at 30 Hz, 3g. In vivo, pQCT analyses of the tibiae were performed at the start of the experiment and after 4 and 8 weeks. After 8 weeks the tibiae were excised for histomorphometric and for in vitro pQCT analyses. In the SHAM-V group, vibration had no effect upon the different bone parameters. In the OVX-V group, vibration induced a significant increase compared to the OVX group of the cortical and medullary areas (P < 0.01) and of the periosteal (P < 0.01) and endosteal (P < 0.05) perimeters at the 3 g vibration. The strain strength index increased in the OVX-V group significantly (P < 0.01) at the higher vibration. The results showed that low-amplitude, high-frequency whole-body vibration is anabolic to bone in OVX animals. The osteogenic potential is limited to the modeling of the bone cortex and depends on the amplitude of the vibration.





Similar content being viewed by others
References
Rubin CT, Mcleod KJ, Bain SD (1990) Functional strains and cortical bone adaptation: epigenetic assurance of skeletal integrity. J Biomech 23(Suppl 1):43–54
Rubin CT, Sommerfield DW, Judex S, Qin Y (2001) Inhibition of osteopenia by low magnitude, high frequency mechanical stimuli. Drug Discov Today 6:848–858
Rubin CT, Turner AS, Bain S, Mallinckrodt C, McLeod K (2001) Anabolism. Low mechanical signals strengthen long bones. Nature 412:603–604
Jankovich JP (1972) The effects of mechanical vibration on bone development in the rat. J Biomech 5:241–250
Rubin C, Turner AS, Mallinckrodt C, Jerome C, Mcleod K, Bain S (2002) Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone 30:445–52
Judex S, Zhong N, Squire ME, Ye K, Donahue LR, Hadjiargyrou M, Rubin CT (2005) Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J Cell Biochem 94:982–994
Tanaka SM, Li J, Duncan RL, Yokota H, Burr DB, Turner CH (2003) Effects of broad frequency vibration on cultured osteoblasts. J Biomech 36:73–80
Usui Y, Zerwekh JE, Vanharanta H, Ashman RB, Mooney V (1989) Different effects of mechanical vibration on bone ingrowth into porous hydroxyapatite and fracture healing in a rabbit model. J Orthop Res 7:559–567
Tanaka SM, Alam I, Turner CH (2003) Stochastic resonance in osteogenic response to mechanical loading. FASEB J 17:313–314
Oxlund BS, Ortoft G, Andreassen TT, Oxlund H (2003) Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone 32:69–77
Flieger J, Karachalios TH, Khaldi L, Raptou P, Lyritis G (1998) Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif Tissue Int 63:510–514
Frost HM (1987) Bone mass and the mechanostat: a proposal. Anat Rec 219:1–9
Frost HM (1983) A determinant of bone architecture. The minimum effective strain. Clin Orthop 175:286–292
Frost HM (1987) The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and non mechanical agents. Bone Miner 2:73–85
Lanyon LE, Sherry TM (2001) Perspective. Postmenopausal osteoporosis as a failure of bone’s adaptation to functional loading: a hypothesis. J Bone Miner Res 16:1937–1947
Lanyon LE, Armstrong V, Ong D, Zaman G, Price J (2004) Is estrogen receptor α key to controlling bones’ resistence to fracture? J Endocrinol 182:183–191
Jessop HL, Sjoberg M, Cheng MZ, Zaman G, Weeler-Jones CP, Lanyon LE (2001) Mechanical strain and estrogen activate estrogen receptor alpha in bone cells. J Bone Miner Res 16:1045–1055
Ehrlich PJ, Noble BS, Jessop HL, Stevens HY, Mosley JR, Lanyon LE (2002) The effect of in vivo mechanical loading on estrogen receptor alpha expression in rat ulnar osteocytes. J Bone Miner Res 17:1646–1655
Lee K, Jessop HL, Suswillo R, Zaman G, Lanyon L (2003) Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature 424:389
Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, Lanyon LL (2006) Osteocytes use estrogen receptor α to respond to strain but their ERα content is regulated by estrogen. J Bone Miner Res 21:1297–1306
Bass SL, Saxon LK, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S (2002) The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res 17:2274–2280
Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK (2003) Bone loss and bone size after menopause. N Engl J Med 349:327–334
Vanderschueren D, Venken K, Ophoff J, Bouillon R, Boonen S (2006) Sex steroids and the periosteum: reconsidering the roles of androgens and estrogens in periosteal expansion. J Clin Endocrinol Metab 91:378–382
Duan Y, Beck TJ, Wang XF, Seeman E (2003) Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res 18:1766–1774
Bouillon R, Bex M, Vanderschueren D, Boonen S (2004) Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 89:6025–6029
Saxon LK, Turner CH (2005) Estrogen receptor β: the antimechanostat? Bone 36:185–192
Westerlind KC, Wronski TJ, Ritman EL, Luo ZP, An KN, Bell NH, Turner RT (1997) Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci USA 94:4199–4204
Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C (2006) Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res 21:1464–1474
Torvinen S, Kannus P, Sievanen H, Jarvinen TA, Pasanen M, Kontulainen S, Nenonen A, Jarvinen TL, Paakkala T, Jarvinen M, Vuori I (2003) Effect of 8-month vertical whole-body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res 18: 876–884
Rabkin BA, Szivek JA, Schonfeld JE, Halloran BP (2001) Long term measurement of bone strain in vivo: the rat tibia. J Biomed Mater Res B Appl Biomater 58:277–281
Ioannidis GV, Skarantavos G, Raptou P, Khaldi L, Karachalios TH, Lyritis GP (2000) Whole-body vibrations induce periosteal bone formation in ovariectomised rats. J Musculoskelet Neuronal Interact 1:70–71
Castillo AB, Alam I, Tanaka SM, Levenda J, Li J, Warden SJ, Turner CH (2006) Low-amplitude, broad-frequency vibration effects on cortical bone in mice. Bone 39:1087–1096
Saxon LK, Turner CH (2006) Low-dose estrogen treatment suppresses periosteal bone formation in response to mechanical loading. Bone 39:1261–1267
Saxon LK, Robling AG, Castillo AB, Mohan S, Turner CH (2007) The skeletal responsiveness to mechanical loading is enhanced in mice with a null mutation in estrogen receptor-beta. Am J Physiol Endocrinol Metab 293:E484–E491
Seeman E (2007) The periosteum—a surface for all seasons. Osteoporos Int 18:123–128
Garman R, Gaudette G, Donahue LR, Rubin C, Judex S (2007) Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res 25:732–740
Judex S, Lei X, Han D, Rubin C (2007) Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech 40:1333–1339
Christiansen BA, Silva MJ (2006) The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann Biomed Eng 34:1149–1156
Warden SJ, Turner CH (2004) Mechanotransduction in the cortical bone is most efficient at loading frequencies of 5–10 Hz. Bone 34:261–270
Beamer WG, Donahue LR, Rosen CJ, Baylink DJ (1996) Genetic variability in adult bone density among inbred strains of mice. Bone 18:397–403
Iida H, Fukuda S (2002) Age-related changes in bone mineral density, cross-sectional area and strength at different skeletal sites in male rats. J Vet Med Sci 64:29–34
Allen MR, Hock JM, Burr DB (2004) Periosteum: biology, regulation, and response to osteoporosis therapies. Bone 35:1003–1012
Cardinale M, Rittweger J (2006) Vibration exercise makes your muscles and bones stronger: fact or fiction? J Br Menopause Soc 12:12–18
Griffin MJ (2004) Minimum health and safety requirements for workers exposed to hand-transmitted vibration and whole-body vibration in the European Union: a review. Occup Environ Med 61:387–397
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rubinacci, A., Marenzana, M., Cavani, F. et al. Ovariectomy Sensitizes Rat Cortical Bone to Whole-Body Vibration. Calcif Tissue Int 82, 316–326 (2008). https://doi.org/10.1007/s00223-008-9115-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-008-9115-8