Abstract
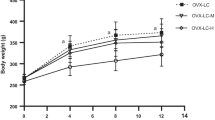
Long chain polyunsaturated fatty acids (LCPUFAs) are involved in the regulation of bone metabolism. Increased dietary consumption of n-3, and possibly some n-6, LCPUFAs may limit postmenopausal bone loss. The aim of this study was to determine the effects on bone of specific fatty acids within the n-3 and n-6 LCPUFA families in ovariectomized (OVX) rats. Rats were OVX or sham-operated and fed either a control diet (OVX and sham) or a diet supplemented with 0.5 g/kg body weight/day of γ-linolenic (GLA), eicosapentaenoic (EPA), docosahexaenoic (DHA) ethyl esters or a mixture of all three (MIX) for 16 weeks. Bone mineral content (BMC), area, and density and plasma concentrations of insulin-like growth factor-I, vitamin D, selected biochemical markers of bone metabolism, and parathyroid hormone (PTH) were determined. The OVX-induced decrease in lumbar spine BMC was significantly attenuated by DHA but not by EPA or GLA supplementation or supplementation with a mixture of all three LCPUFAs. Endosteal circumferences of tibiae were significantly greater in DHA and EPA compared to OVX. Plasma C-terminal telopeptide of type I collagen and osteocalcin concentrations were not significantly different in the DHA group compared to OVX. Femur BMC decreased by a significantly greater amount in GLA than OVX, and final plasma PTH concentrations were significantly higher in GLA compared to all other groups. In conclusion, DHA ameliorated OVX-induced bone mineral loss. GLA exacerbated post-OVX bone mineral loss, possibly as a result of PTH-induced bone catabolism.
Similar content being viewed by others
References
Kruger M, Horrobin D (1997) Calcium metabolism, osteoporosis and essential fatty acids. A review. Prog Lipid Res 36:131–151
Watkins B, Seifert M (1996) Food lipids and bone health. In: McDonald R, Min D (eds) Food lipids and health. Marcel Dekker, New York
Das UN (2000) Essential fatty acids and osteoporosis. Nutrition 16:386–390
Raisz LG, Pilbeam CC, Fall PM (1993) Prostaglandins—mechanisms of action and regulation of production in bone. Osteoporos Int 3:S136–S140
Conconi MT, Tommasini M, Baiguera S, De Coppi P, Parnigotto PP, Nussdorfer GG (2002) Effects of prostaglandins E1 and E2 on the growth and differentiation of osteoblast-like cells cultured in vitro. Int J Mol Med 10:451–456
Yoshiko Y, Gonsalves P, Hiyama S, Uchida T, Maeda N, Aubin JE (2003) Activation of prostaglandin E receptor EP2 during the osteoprogenitor proliferation-differentiation transition is the major pathway mediating the anabolic effects of PGE2 on osteoblast development in rat calvaria cell cultures. Bone 32:S139–S139
Collins DA, Chambers TJ (1991) Effect of prostaglandins E1, E2, and F2α on osteoclast formation in mouse bone-marrow cultures. J Bone Miner Res 6:157–164
Ono K, Kaneko H, Choudhary S, Pilbeam CC, Lorenzo JA, Akatsu T, Kugai N, Raisz LG (2005) Biphasic effect of prostaglandin E2 on osteoclast formation in spleen cell cultures: role of the EP2 receptor. J Bone Miner Res 20:23–29
Yun AJ, Lee PY (2004) Maladaptation of the link between inflammation and bone turnover may be a key determinant of osteoporosis. Med Hypotheses 63:532–537
Teitelbaum SL (2004) Postmenopausal osteoporosis, T cells, and immune dysfunction. Proc Natl Acad Sci USA 101:16711–16712
Johnson MM, Swan DD, Surette ME, Stegner J, Chilton T, Fonteh AN, Chilton FH (1997) Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J Nutr 127:1435–1444
Miller CC, Tang W, Ziboh VA, Fletcher MP (1991) Dietary supplementation with ethyl-ester concentrates of fish oil (n-3) and borage oil (n-6) polyunsaturated fatty-acids induces epidermal generation of local putative antiinflammatory metabolites. J Invest Dermatol 96:98–103
Kumar GS, Das UN, Kumar KV, Madhavi N, Das NP, Tan BKH (1992) Effect of n-6 and n-3 fatty-acids on the proliferation of human-lymphocytes and their secretion of TNF-alpha and IL-2 invitro. Nutr Res 12:815–823
Furse RK, Rossetti RG, Zurier RB (2001) Gamma-linolenic acid, an unsaturated fatty acid with anti-inflammatory properties, blocks amplification of IL-1 beta production by human monocytes. J Immunol 167:490–496
Furse RK, Rossetti RG, Seiler CM, Zurier RB (2002) Oral administration of gamma-linolenic acid, an unsaturated fatty acid with anti-inflammatory properties, modulates interleukin-10 production by human monocytes. J Clin Immunol 22:83–91
Decaterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Libby P (1994) The omega-3-fatty-acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial-cells. Arterioscler Thromb 14:1829–1836
Soyland E, Lea T, Sandstad B, Drevon A (1994) Dietary supplementation with very long-chain n-3 fatty-acids in man decreases expression of the interleukin-2 receptor (Cd25) on mitogen-stimulated lymphocytes from patients with inflammatory skin diseases. Eur J Clin Invest 24:236–242
Danno K, Ikai K, Imamura S (1993) Antiinflammatory effects of eicosapentaenoic acid on experimental skin inflammation models. Arch Dermatol Res 285:432–435
Robinson DR, Xu LL, Tateno S, Guo MY, Colvin RB (1993) Suppression of autoimmune-disease by dietary n-3 fatty-acids. J Lipid Res 34:1435–1444
Weiss LA, Barrett-Connor E, von Muhlen D (2005) Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am J Clin Nutr 81:934–938
Kruger M, Coetzer H, de Winter R, Claassen N (1995) Eicosapentaenoic acid and docosahexaenoic acid supplementation increases calcium balance. Nutr Res 15:211–219
Kruger M, Claassen N, Schlemmer C, Coetzer H (1999) Essential fatty acid supplementation: effect on oestrogen deficiency induced bone loss in the female rat. Calcif Tissue Int 64:63
Weiler HA (2000) Dietary supplementation of arachidonic acid is associated with higher whole body weight and bone mineral density in growing pigs. Pediatr Res 47:692–697
Kruger M, Coetzer H, de Winter R, Gericke G, van Papendorp D (1998) Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging Clin Exp Res 10:385–394
Claassen N, Coetzer H, Steinmann C, Kruger M (1995) The effect of different n-6/n-3 essential fatty acid ratios on calcium balance and bone in rats. Prostaglandins Leukot Essent Fatty Acids 53:13–19
Claassen N, Potgieter HC, Seppa M, Vermaak WJH, Coetzer H, Vanpapendorp DH, Kruger MC (1995) Supplemented gamma-linolenic acid and eicosapentaenoic acid influence bone status in young male rats—effects on free urinary collagen cross-links, total urinary hydroxyproline, and bone calcium content. Bone 16:S385–S392
Watkins BA, Shen CL, Allen KGD, Seifert MF (1996) Dietary (n-3) and (n-6) polyunsaturates and acetylsalicylic acid alter ex vivo PGE2 biosynthesis, tissue IGF-I levels, and bone morphometry in chicks. J Bone Miner Res 11:1321–1332
Poulsen RC, Kruger MC (2006) Detrimental effect of eicosapentaenoic acid supplementation on bone following ovariectomy in rats. Prostaglandins Leukot Essent Fatty Acids 75:419–427
Gnudi SRG, Mongiorgi R, Fini M, Zati A, Figus E, Monti S, Ripamonti C (1993) Evaluation of an experimental model of osteoporosis induced in the female rat through ovariectomy. Boll Soc Ital Biol Sper 69:453–460
Kruger MC, Schollum LM (2005) Is docosahexaenoic acid more effective than eicosapentaenoic acid for increasing calcium bioavailability? Prostaglandins Leukot Essent Fatty Acids 73:327–334
Schlemmer C, Coetzer H, Claassen N, Kruger M (1999) Oestrogen and essential fatty acid supplementation corrects bone loss due to ovariectomy in the female Sprague Dawley rat. Prostaglandins Leukot Essent Fatty Acids 61:381–390
Baggio B, Budakovic A, Nassuato MA, Vezzoli G, Manzato E, Luisetto G, Zaninotto M (2000) Plasma phospholipid arachidonic acid content and calcium metabolism in idiopathic calcium nephrolithiasis. Kidney Int 58:1278–1284
Shen C-L, Yeh J, Rasty J, Li Y, Watkins B (2006) Protective effect of dietary long-chain n-3 polyunsaturated fatty acids on bone loss in gonad-intact middle-aged male rats. Br J Nutr 95:462–468
National Research Council (1995) Nutrient requirements of laboratory animals, 4th rev ed. National Academy Press, Washington DC
Rahman M, Bhattacharya A, Banu J, Fernandes G (2005) Reduced bone loss by docosahexaenoic acid (DHA) than eicosapentaenoic acid (EPA) in ovariectomised mice. J Bone Miner Res 20:S127
Li Y, Reinwald S, Seifert M, Watkins B (2003) Dietary docosahexaenoic acid supplementation maintains bone mass in ovariectomised rats. Exp Biol Abstracts B321:188.110
Watkins BA, Li Y, Seifert MF (2006) Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: actions on bone mineral and serum biomarkers in ovariectomized rats. J Nutr Biochem 17:282–289
Andersson S, Holmberg I, Wikvall K (1983) 25-Hydroxylation of C27-steroids and vitamin D3 by a constitutive cytochrome P-450 from rat liver microsomes. J Biol Chem 258:6777–6781
Roborgh JR, de Man TJ (1960) The hypercalcemic activity of dihydrotachysterol2 and dihydrotachysterol3 and of the vitamins D2 and D3 after intravenous injection of the aqueous preparations. II: Comparative experiments on rats. Biochem Pharmacol 3:277–282
Nakane M, Fey TA, Dixon DB, Ma JL, Brune ME, Li YC, Wu-Wong JR (2006) Differential effects of vitamin D analogs on bone formation and resorption. J Steroid Biochem Mol Biol 98:72–77
Sakaguchi K, Morita I, Murota S (1994) Eicosapentaenoic acid inhibits bone loss due to ovariectomy in rats. Prostaglandins Leukot Essent Fatty Acids 50:81–84
Leonard F, Haag M, Kruger M (2001) Modulation of intestinal vitamin D receptor availability and calcium ATPase activity by essential fatty acids. Prostaglandins Leukot Essent Fatty Acids 64:147–150
Brandi M, Fitzpatrick L, Coon H, Aurbach G (1986) Bovine parathyroid cells: cultures maintained for more than 140 population doublings. Proc Natl Acad Sci USA 83:1709–1713
Shemerdiak W, Kukreja S, Johnson P (1981) Effect of prostaglandin E1, E2 or indomethacin on serum parathyroid hormone and calcitonin in the rat. Prostaglandins Med 6:641–645
Qin L, Raggatt LJ, Partridge NC (2004) Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab 15:60–65
Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S, Yamaguchi A (1997) Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest 99:2961–2970
Acknowledgments
This study was funded by Fonterra Brands Ltd., New Zealand. R. C. P. was the recipient of a TAD scholarship from the Tertiary Education Commission, New Zealand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poulsen, R.C., Firth, E.C., Rogers, C.W. et al. Specific Effects of γ-Linolenic, Eicosapentaenoic, and Docosahexaenoic Ethyl Esters on Bone Post-ovariectomy in Rats. Calcif Tissue Int 81, 459–471 (2007). https://doi.org/10.1007/s00223-007-9080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-007-9080-7