Abstract
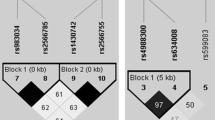
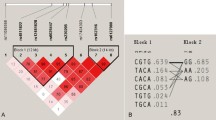
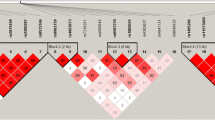
We investigated whether polymorphisms in PTHR1 are associated with bone mineral density (BMD), to determine whether the association of this gene with BMD was due to effects on attainment of peak bone mass or effects on subsequent bone loss. The PTHR1 gene, including its 14 exons, their exon-intron boundaries, and 1,500 bp of its promoter region, was screened for polymorphisms by denaturing high-performance liquid chromatography (dHPLC) and sequencing in 36 osteoporotic cases. Eleven single-nucleotide polymorphisms (SNPs), one tetranucleotide repeat, and one tetranucleotide deletion were identified. A cohort of 634 families, including 1,236 men (39%) and 1,926 women (61%) ascertained with probands with low BMD (Z< −2.0) and the Children in Focus subset of the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort (785 unrelated individuals, mean age 118 months), were genotyped for the five most informative SNPs (minor allele frequency >5%) and the tetranucleotide repeat. In our osteoporosis families, association was noted between lumbar spine BMD and alleles of a known functional tetranucleotide repeat (U4) in the PTHR1 promoter region (P = 0.042) and between two and three marker haplotypes of PTHR1 polymorphisms with lumbar spine, femoral neck, and total hip BMD (P = 0.021–0.047). This association was restricted to the youngest tertile of the population (age 16–39 years, P = 0.013–0.048). A similar association was found for the ALSPAC cohort: two marker haplotypes of SNPs A48609T and C52813T were associated with height (P = 0.006) and total body less head BMD (P = 0.02), corrected for age and gender, confirming the family findings. These findings suggest a role for PTHR1 variation in determining peak BMD.

Similar content being viewed by others
References
Pocock N, Eisman J, Hopper J, Yeates M, Sambrook P, Eberl S (1987) Genetic determinants of bone mass in adults. A twin study. J Clin Invest 80:706–710
Nguyen TV, Howard GM, Kelly PJ, Eisman JA (1998) Bone mass, lean mass, and fat mass: same genes or same environments? Am J Epidemiol 147:3–16
Flicker L, Hopper J, Rodgers L, Kaymakci B, Green R, Wark J (1995) Bone density determinants in elderly women: a twin study. J Bone Miner Res 10:1607–1613
Gueguen R, Jouanny P, Guillemin F, Kuntz C, Pourel J, Siest G (1995) Segregation analysis and variance components analysis of bone mineral density in healthy families. J Bone Miner Res 10:2017–2022
Arden NK, Baker J, Hogg C, Baan K, Spector TD (1996) The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. J Bone Miner Res 11:530–534
Duncan E, Cardon L, Sinsheimer J, Wass J, Brown M (2003) Site and gender specificity of inheritance of bone mineral density. J Bone Miner Res 18:1531–1538
Abecasis G, Cardon L, Cookson W (2000) A general test of association for quantitative traits in nuclear families. Am J Hum Genet 66:279–292
Deng H, Xu F, Huang Q, Shen H, Deng H, Conway T, Liu Y, Liu Y, Li J, Zhang H, Davies K, Recker R (2002) A whole-genome linkage scan suggests several genomic regions potentially containing quantitative trait loci for osteoporosis. J Clin Endocrinol Metab 87:5151–5159
Seeman E, Tsalamandris C, Formica C, Hopper JL, McKay J (1994) Reduced femoral neck bone density in the daughters of women with hip fractures: the role of low peak bone density in the pathogenesis of osteoporosis. J Bone Miner Res 9:739–743
Seeman E, Hopper JL, Bach LA, Cooper ME, Parkinson E, McKay J, Jerums G (1989) Reduced bone mass in daughters of women with osteoporosis. N Engl J Med 320:554–558
Duncan E, Brown M, Sinsheimer J, Bell J, Carr A, Wordsworth B, Wass J (1999) Suggestive linkage of the parathyroid receptor type 1 to osteoporosis. J Bone Miner Res 14:1993–1999
Wilson SG, Reed PW, Bansal A, Chiano M, Lindersson M, Langdown M, Prince RL, Thompson D, Thompson E, Bailey M, Kleyn PW, Sambrook P, Shi MM, Spector TD (2003) Comparison of genome screens for two independent cohorts provides replication of suggestive linkage of bone mineral density to 3p21 and 1p36. Am J Hum Genet 72:144–155
Wynne F, Drummond FJ, Daly M, Brown M, Shanahan F, Molloy MG, Quane KA (2003) Suggestive linkage of 2p22–25 and 11q12–13 with low bone mineral density at the lumbar spine in the Irish population. Calcif Tissue Int 72:651–658
Ioannidis JP, Ng MY, Sham PC, Zintzaras E, Lewis CM, Deng HW, Econs MJ, Karasik D, Devoto M, Kammerer CM, Spector T, Andrew T, Cupples LA, Duncan EL, Foroud T, Kiel DP, Koller D, Langdahl B, Mitchell BD, Peacock M, Recker R, Shen H, Sol-Church K, Spotila LD, Uitterlinden AG, Wilson SG, Kung AW, Ralston SH (2007) Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J Bone Miner Res 22:173–183
Kruse K, Schutz C (1993) Calcium metabolism in the Jansen type of metaphyseal dysplasia. Eur J Pediatr 152:912–915
Schipani E, Langman CB, Parfitt AM, Jensen GS, Kikuchi S, Kooh SW, Cole WG, Juppner H (1996) Constitutively activated receptors for parathyroid hormone and parathyroid hormone-related peptide in Jansen’s metaphyseal chondrodysplasia. N Engl J Med 335:708–714
Jobert A-S, Zhang P, Couvineau A, Bonaventure J, Roume J, Le Merrer M, Silve C (1998) Absence of functional receptors for parathryoid hormone and parathyroid hormone-related peptide in Blomstrand chondrodysplasia. J Clin Invest 102:34–30
Minagawa M, Yasuda T, Watanabe T, Minamitani K, Takahashi Y, Goltzman D, White J, Hendy G, Kohno Y (2002) Association between AAAG repeat polymorphism in the P3 promoter of the human parathyroid hormone (PTH)/PTH related peptide receptor gene and adult height, urinary pyridinoline excretion and promoter activity. J Clin Endocrinol Metab 87:1791–1796
Scillitani A, Jang C, Wong B, Hendy G, Cole D (2006) A functional polymorphism in the PTHR1 promoter region is associated with adult height and BMD measured at the femoral neck in a large cohort of young Caucasian women. Hum Genet 119:416–421
Jones RW, Ring S, Tyfield L, Hamvas R, Simmons H, Pembrey M, Golding J (2000) A new human genetic resource: a DNA bank established as part of the Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC). Eur J Hum Genet 8:653–660
Ralston SH, Galway N, MacKay N, Albagha OME, Cardon LR, Compston JE, Cooper C, Duncan EL, Keen RW, Langdahl B, McLellan A, O’Riordan JLH, Pols HA, Reid DM, Uitterlinden AG, Wass JA, Bennett ST (2004) Loci for regulation of BMD in men and women: the FAMOS study. Calcif Tissue Int 74:S39–S40
Golding J, Pembrey M, Jones R (2001) ALSPAC – the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 15:74–87
Tanner JM (1986) Normal growth and techniques of growth assessment. Clin Endocrinol Metab 15:411–451
Tobias JH, Steer CD, Emmett PM, Tonkin RJ, Cooper C, Ness AR (2005) Bone mass in childhood is related to maternal diet in pregnancy. Osteoporos Int 16:1731–1741
Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin – rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101
Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363
Stephens M, Smith N, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989
Nyholt DR (2004) A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 74:765–769
Lei SF, Wang YB, Liu MY, Mo XY, Deng HW (2005) The VDR, COL1A1, PTH, and PTHR1 gene polymorphisms are not associated with bone size and height in Chinese nuclear families. J Bone Miner Metab 23:501–505
Liu PY, Zhang YY, Lu Y, Long JR, Shen H, Zhao LJ, Xu FH, Xiao P, Xiong DH, Liu YJ, Recker RR, Deng HW (2005) A survey of haplotype variants at several disease candidate genes: the importance of rare variants for complex diseases. J Med Genet 42:221–227
Wellcome Trust Case-Control Consortium (2007) Genomewide association study of 14,000 cases of seven common diseases and 3000 controls. Nature 447:661–683
Acknowledgements
We are extremely grateful to all the families from the ALSPAC and FAMOS cohorts who took part in this study. With respect to the ALSPAC participants, we thank the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The UK Medical Research Council, the Wellcome Trust, and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, and MAB and CV will serve as guarantors for the contents. This research was specifically funded by the Arthritis Research Campaign (UK) and the National Health and Medical Research Council (Australia); E. L. D. was funded by Action Research (UK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilariño-Güell, C., Miles, L.J., Duncan, E.L. et al. PTHR1 Polymorphisms Influence BMD Variation through Effects on the Growing Skeleton. Calcif Tissue Int 81, 270–278 (2007). https://doi.org/10.1007/s00223-007-9072-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-007-9072-7