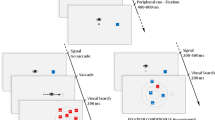
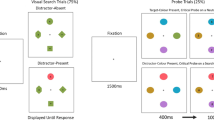
Abstract
To gain insight into how human observers select items in the visual field we pitted two attentional biases against one another in a single free choice design. The first bias is the nasal-temporal asymmetry during free choice tasks, where observers tend to choose targets that appear in their temporal hemifield over targets appearing in their nasal hemifield. The second is the choice bias found in studies of attentional priming. When observers have to select between a stimulus that shares features with a preceding target and a stimulus sharing features with previous distractors, they have a strong tendency to choose the preceding search target and this bias increases the more often the same search is repeated. Our results show that both biases affect saccadic choice, but they also show that the nasal-temporal bias can modulate the strength of the priming effects, but not vice versa. The priming effect was stronger for stimuli appearing in the temporal than in the nasal hemifield, but the nasal-temporal bias was similar for primed and unprimed targets. Additionally, our findings are the first to show how search repetition leads to faster saccades. The observed difference between the effects of the NTA and priming biases may reflect the difference in neural mechanisms thought to be behind these biases and that biases at lower levels may outrank higher-level biases, at least in their effect on visual attention.





Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study will be available from the corresponding author upon request.
Notes
Past evidence has shown no left–right asymmetry in saccades that could potentially affect performance at a saccade choice task (see, e.g., Honda 2002; Tagu et al. 2020; Vergilino-Perez et al. 2012), so although we only covered the left eye, we are quite confident that the left–right differences we observe in the current study reflect naso-temporal asymmetries in choice.
Although the difference in the luminance of the red and green stimuli is considerable it could not have affected the results because the distributions of the colours were perfectly balanced between all conditions in the experiment.
It is not possible to use glmer() here, because there is no grouping variable that can be used as factor.
When the effects of repetition in the whole data set are measured, it is important to note that the same colour combination is repeated twice, occurring in each of the three streak lengths; 4 repetitions occur for streak lengths 4 and 6 repetitions occur only when the streak length is 6. Therefore, there is more data behind 2 repetitions than 4 and more data behind 4 repetitions than 6 repetitions.
References
Anderson SJ, Mullen KT, Hess RF (1991) Human peripheral spatial resolution for achromatic and chromatic stimuli: limits imposed by optical and retinal factors. J Physiol 442(1):47–64. https://doi.org/10.1113/jphysiol.1991.sp018781
Ásgeirsson ÁG, Kristjánsson Á (2019) Attentional priming does not enable observers to ignore salient distractors. Visual Cogn 27(5–8):595–608
Ásgeirsson ÁG, Kristjánsson Á, Bundesen C (2014) Independent priming of location and color in identification of briefly presented letters. Atten Percept Psychophys 76:40–48
Bates D (2015) lme4: mixed-effects modelling with R. http://lme4.r-forge.r-project.org/book/. Accessed 29 Aug 2018
Berger A, Henik A (2000) The endogenous modulation of IOR is nasal-temporal asymmetric. J Cogn Neurosci 12(3):421–428
Bichot NP, Schall JD (2002) Priming in macaque frontal cortex during popout visual search: feature-based facilitation and location-based inhibition of return. J Neurosci 22(11):4675–4685
Bompas A, Sumner P (2008) Sensory sluggishness dissociates saccadic, manual, and perceptual responses: an S-cone study. J vis 8(8):1–13
Bompas A, Sterling T, Rafal RD, Sumner P (2008) Naso-temporal asymmetry for signals invisible to the retinotectal pathway. J Neurophysiol 100(1):412–421
Brascamp JW, Blake R, Kristjánsson Á (2011) Deciding where to attend: priming of pop-out drives target selection. J Exp Psychol Human 37(6):1700–1707. https://doi.org/10.1037/a0025636
Bravo MJ, Nakayama K (1992) The role of attention in different visual-search tasks. Percept Psychophys 51(5):465–472
Brinkhuis MA, Kristjánsson Á, Harvey BM, Brascamp JW (2020) Temporal characteristics of priming of attention shifts are mirrored by BOLD response patterns in the frontoparietal attention network. Cereb Cortex 30(4):2267–2280
Bundesen C (1990) A theory of visual attention. Psychol Rev 97(4):523
Connolly M, Van Essen D (1984) The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. J Comp Neurol 226(4):544–564
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3(3):201–215
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300(1):5–25
Delinte A, Gomez C, Decostre M, Crommelinck M, Roucoux A (2002) Amplitude transition function of human express saccades. Neurosci Res 42:21–34
Desimone R, Duncan J (1995) Neural mechanisms of selective visual attention. Annu Rev Neurosci 18(1):193–222
Deubel H, Schneider WX (1996) Saccade target selection and object recognition: evidence for a common attentional mechanism. Vis Res 36(12):1827–1838
Driver J (2001) A selective review of selective attention research from the past century. Brit J Psychol 92(1):53–78
Fischer B, Weber H (1992) Characteristics of “anti” saccades in man. Exp Brain Res 89(2):415–424
Galley N (1989) Saccadic eye movement velocity as an indicator of (de) activation: A review and some speculations. J Psychophysiol 3:229–244
Goldberg ME, Wurtz RH (1972) Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol 35(4):560–574
Honda H (2002) Idiosyncratic left-right asymmetries of saccadic latencies: Examination in a gap paradigm. Vis Res 42(11):1437–1445
Hubel DH, LeVay S, Wiesel TN (1975) Mode of termination of retinotectal fibers in macaque monkey: an autoradiographic study. Brain Res 96(1):25–40
Huestegge L, Herbort O, Gosch N, Kunde W, Pieczykolan A (2019) Free-choice saccades and their underlying determinants: Explorations of high-level voluntary oculomotor control. J vis 19(3):1–15. https://doi.org/10.1167/19.3.14
Itaya SK, Van Hoesen GW (1983) Retinal projections to the inferior and medial pulvinar nuclei in the old-world monkey. Brain Res 269(2):223–230
Jóhannesson ÓI, Kristjánsson Á (2013) Violating the main sequence: asymmetries in saccadic peak velocities for saccades into the temporal versus nasal hemifields. Exp Brain Res 227(1):101–110. https://doi.org/10.1007/s00221-013-3490-8
Jóhannesson ÓI, Ásgeirsson ÁG, Kristjánsson Á (2012) Saccade performance in the nasal and temporal hemifields. Exp Brain Res 219(1):107–120. https://doi.org/10.1007/s00221-012-3071-2
Jóhannesson ÓI, Edelman JA, Sigurþórsson BD, Kristjánsson Á (2018a) Effects of saccade training on express saccade proportions, saccade latencies, and peak velocities: an investigation of nasal/temporal differences. Exp Brain Res 236(5):1251–1262. https://doi.org/10.1007/s00221-018-5213-7
Jóhannesson ÓI, Tagu J, Kristjánsson Á (2018b) Asymmetries of the visual system and their influence on visual performance and oculomotor dynamics. European J Neurosci 48(11):3426–3445. https://doi.org/10.1111/ejn.14225
Jonikaitis D, Klapetek A, Deubel H (2017) Spatial attention during saccade decisions. J Neurophysiol 118(1):149–160
Koller K, Rafal RD (2019) Saccade latency bias toward temporal hemifield: evidence for role of retinotectal tract in mediating reflexive saccades. Neuropsychologia 128:276–281. https://doi.org/10.1016/j.neuropsychologia.2018.01.028
Kowler E, Anderson E, Dosher B, Blaser E (1995) The role of attention in the programming of saccades. Vis Res 35(13):1897–1916
Krauzlis RJ, Lovejoy LP, Zénon A (2013) Superior colliculus and visual spatial attention. Annu Rev Neurosci 36(1):165–182. https://doi.org/10.1146/annurev-neuro-062012-170249
Kristjánsson Á (2006) Rapid learning in attention shifts: a review. Visual Cogn 13(3):324–362
Kristjánsson Á (2011) The intriguing interactive relationship between visual attention and saccadic eye movements. In: Liversedge L, Gilchrist ID, Everling S (eds) Oxford handbook of eye movements, vol 1, 1st edn. Oxford University Press, Oxford, pp 455–470
Kristjánsson Á, Campana G (2010) Where perception meets memory: A review of repetition priming in visual search tasks. Atten Percept Psychophys 72(1):5–18. https://doi.org/10.3758/APP.72.1.5
Kristjánsson Á, Ásgeirsson ÁG (2019) Attentional priming: recent insights and current controversies. Curr Opin Psychol 29:71–75
Kristjánsson Á, Egeth H (2020) How feature integration theory integrated cognitive psychology, neurophysiology, and psychophysics. Atten Percept Psychophys 82:7–23
Kristjánsson Á, Chen Y, Nakayama K (2001) Less attention is more in the preparation of antisaccades, but not prosaccades. Nature Neurosci 4:1037–1042
Kristjánsson Á, Wang D, Nakayama K (2002) The role of priming in conjunctive visual search. Cognition 85(1):37–52. https://doi.org/10.1016/S0010-0277(02)00074-4
Kristjánsson A, Vandenbroucke M, Driver J (2004) When pros become cons for anti-versus prosaccades: factors with opposite or common effects on different saccade types. Exp Brain Res 155(2):231–244
Kristjánsson Á, Vuilleumier P, Schwartz S, Macaluso E, Driver J (2007) Neural basis for priming of pop-out during visual search revealed with fMRI. Cereb Cortex 17(7):1612–1624
Maljkovic V, Nakayama K (1994) Priming of pop-out: I. Role of Features Memory Cogn 22(6):657–672
McPeek RM, Maljkovic V, Nakayama K (1999) Saccades require focal attention and are facilitated by a short-term memory system. Vis Res 39(8):1555–1566. https://doi.org/10.1016/s0042-6989(98)00228-4
Nakayama K, Maljkovic V, Kristjansson A (2004) Short-term memory for the rapid deployment of visual attention. In: Gazzaniga MS (ed) The cognitive neurosciences. MIT Press, pp 397–408
Osterberg G (1935) Topography of the layer of rods and cones in the human retina. Nyt nordisk forlag, Copenhagen
Peirce J, MacAskill M (2018) Building experiments in PsychoPy. Sage, London
Peirce J, Gray JR, Simpson S, MacAskill M, Höchenberger R, Sogo H, Lindeløv JK (2019) PsychoPy2: experiments in behavior made easy. Behav Res Methods 51(1):195–203
Perry VH, Oehler R, Cowey A (1984) Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience 12(4):1101–1123
Posner M, Cohen Y (1980) Attention and the control of movements. In: Stelmach GE, Requin J (eds) Tutorials in motor behavior. Elsevier/North-Holland, Amsterdam, pp 243–258
R Core Team (2020) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. https://www.R-project.org. Accessed 29 Feb 2020
Rafal RD, Calabresi PA, Brennan CW, Sciolto TK (1989) Saccade preparation inhibits reorienting to recently attended locations. J Exp Psychol Human 15(4):673–685. https://doi.org/10.1037//0096-1523.15.4.673
Rafal RD, Henik A, Smith J (1991) Extrageniculate contributions to reflex visual orienting in normal humans: A temporal hemi- field advantage. J Cogn Neurosci 3(4):322–328
Rizzolatti G, Riggio L, Dascola I, Umiltá C (1987) Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25(1):31–40
Rorden C, Kristjánsson Á, Revill KP, Saevarsson S (2011) Neural correlates of inter-trial priming and role-reversal in visual search. Front Hum Neurosci 5:1–8. https://doi.org/10.3389/fnhum.2011.00151
RStudio Team (2020) RStudio: integrated development for R. RStudio Inc., Boston, MA. http://www.rstudio.com. Accessed 29 Feb 2020
Scolari M, Seidl-Rathkopf KN, Kastner S (2015) Functions of the human frontoparietal attention network: evidence from neuroimaging. Curr Opin Behav Sci 1:32–39
Shurygina O, Kristjánsson Á, Tudge L, Chetverikov A (2019) Expectations and perceptual priming in a visual search task: evidence from eye movements and behavior. J Exp Psychol Human 45(4):489
Sigurdardottir HM, Kristjánsson Á, Driver J (2008) Repetition streaks increase perceptual sensitivity in visual search of brief displays. Visual Cogn 16(5):643–658. https://doi.org/10.1080/13506280701218364
Sylvester R, Josephs O, Driver J, Rees G (2007) Visual fMRI responses in human superior colliculus show a temporal-nasal asymmetry that is absent in lateral geniculate and visual cortex. J Neurophysiol 97(2):1495–1502. https://doi.org/10.1152/jn.00835.2006
Tagu J, Doré-Mazars K, Vergne J, Lemoine-Lardennois C, Vergilino-Perez D (2018a) Recentering bias for temporal saccades only: Evidence from binocular recordings of eye movements. J vis 18(1):1–16. https://doi.org/10.1167/18.1.10
Tagu J, Doré-Mazars K, Vergne J, Lemoine-Lardennois C, Vergilino-Perez D (2018b) Quantifying eye dominance strength—new insights into the neurophysiological bases of saccadic asymmetries. Neuropsychologia 117:530–540. https://doi.org/10.1016/j.neuropsychologia.2018.07.020
Tagu J, Doré-Mazars K, Vergilino-Perez D (2020) Saccade accuracy as an indicator of the competition between functional asymmetries in vision. Exp Brain Res 238(2):411–425. https://doi.org/10.1007/s00221-019-05717-6
Theeuwes J, Van der Burg E (2011) On the limits of top-down control of visual selection. Atten Percept Psychophys 73(7):2092
Tigges J, Tigges M (1981) Distribution of retionfugal and corticofugal axon terminals in the superior colliculus of squirrel monkey. Invest Ophth vis Sci 20:149–158
Toosy AT, Werring DJ, Plant GT, Bullmore ET, Miller DH, Thompson AJ (2001) Asymmetrical activation of human visual cortex demonstrated by functional MRI with monocular stimulation. Neuroimage 14(3):632–641
Tychsen L, Burkhalter A (1997) Nasotemporal asymmetries in V1: ocular dominance columns of infant, adult, and strabismic macaque monkeys. J Comp Neurol 388(1):32–46
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York (ISBN 0-387-95457-0)
Vergilino-Perez D, Fayel A, Lemoine C, Senot P, Vergne J, Doré-Mazars K (2012) Are there any left-right asymmetries in saccade parameters? Examination of latency, gain, and peak velocity. Invest Ophthalmol vis Sci 53(7):3340–3348. https://doi.org/10.1167/iovs.11-9273
Westerberg JA, Maier A, Schall JD (2020) Priming of attentional selection in macaque visual cortex: feature-based facilitation and location-based inhibition of return. Eneuro. https://doi.org/10.1523/ENEURO.0466-19.2020
White BJ, Munoz DP (2011) The superior colliculus. In: Liversedge SP, Gilchrist ID, Everling S (eds) The Oxford handbook of eye movements. Oxford University Press, New York, pp 195–213
Williams C, Azzopardi P, Cowey A (1995) Nasal and temporal retinal ganglion cells projecting to the midbrain: implications for “blindsight.” Neuroscience 65(2):577–586
Funding
ÁK was supported by the Icelandic research fund (#207045-052 and # 173947) and the research fund at the University of Iceland and T was supported by the Icelandic research fund (#206744-051).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Communicated by Francesca Frassinetti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jóhannesson, Ó.I., Kristjánsson, Á. & Tagu, J. Contrasting attentional biases in a saccadic choice task. Exp Brain Res 240, 173–187 (2022). https://doi.org/10.1007/s00221-021-06245-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06245-y